Amino acid insufficiency, and the cellular mechanisms that sense and signal this tissue stress, are linked to immune regulation, wound healing, cell survival and lifespan extension. At low levels, stresses like amino acid insufficiency can induce beneficial effects, akin to exercise that can be damaging at high intensity, but improves muscle strength and fitness at lower levels. Cells detect and respond to stressors by activation of signaling circuits called cellular stress pathways. Like the body’s response to exercise, activation of cellular stress pathways can be physiologically beneficial or potentially damaging, making an understanding of their regulation therapeutically important.
GCN2 is a key protein signaling component of a cellular stress response that is activated when amino acid levels fall below the level required to maintain uninterrupted protein synthesis. Therefore, deciphering GCN2 activation is central to understanding a cell’s response to amino acid stress, including how the limitation of amino acid availability can have therapeutic or life-extending effects. Despite extensive study, the mechanism of GCN2 activation remains unresolved. Recent work has drawn attention to a central role for ribosomes, the cellular protein synthetic machines, as critical sensors of cell stress, but the mechanism by which ribosomes could link amino acid stress to GCN2 activation is unknown. A major limitation to solving this question has been the absence of an experimental system that allows uncoupling and head-to-head comparison of the previously proposed candidates for GCN2 activation.
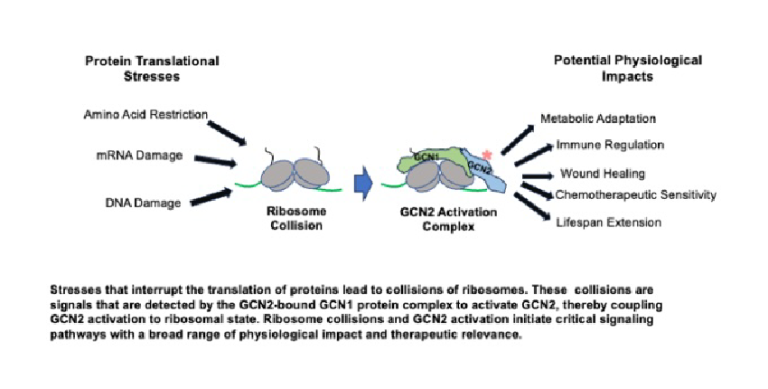
In Zhou et al., we reconstituted the components needed for protein synthesis in a cell-free system and introduced translational stresses under conditions that distinguished between proposed GCN2-activation events. A key tool of this experimental system was the use of halofuginone (HF), a drug that mimics insufficiency of the amino acid proline. Stresses that interrupt protein synthesis trigger collisions between ribosomes, when a trailing ribosome encounters a lead, stalled ribosome on the same mRNA (Fig.1). This study showed that collided ribosome signals were detected by the sensor protein GCN1, which, in turn, enabled the binding and activation of GCN2 on collided ribosomes. This work defines the core requirements of GCN2’s activation, coupling GCN2 activation to ribosomal state.