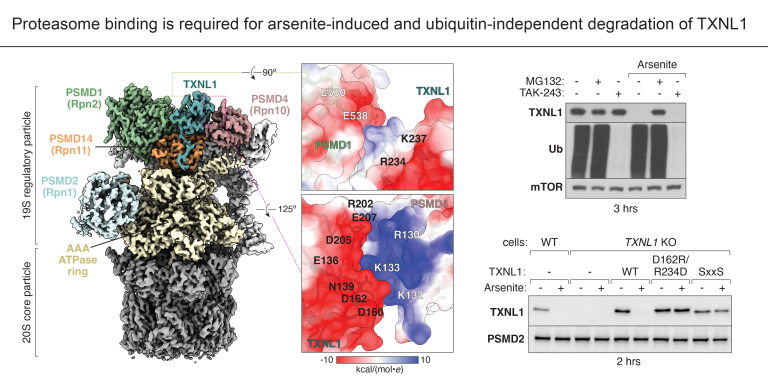
The thioredoxin-like protein TXNL1 is an abundant and conserved thioreductase that stably interacts with proteasomes for poorly understood reasons. Here, the Shao lab, in collaboration with the Elledge and Gu labs, determines the cryo-EM structure of TXNL1 bound to the proteasome. The structure reveals that TXNL1 engages the regulatory particle of the proteasome, which is responsible for recruiting and unfolding protein substrates for degradation. Notably, the placement of TXNL1 above the AAA-ATPase motor, which translocates substrates into the core particle of the proteasome for proteolysis, supports a potential role for TXNL1 in helping to process oxidized proteins for proteasomal degradation. In addition, both the interactions that TXNL1 make with the proteasome as well as its thioreductase activity are required for the degradation of TXNL1 itself upon oxidative stress induced by exposing cells to arsenite. Interestingly, while proteasomes canonically degrade poly-ubiquitylated proteins, the stress-induced degradation of TXNL1 is ubiquitin-independent. These molecular-level insights contribute new understanding into the regulation and mechanisms of proteasomal degradation, a process essential for maintaining cellular homeostasis.