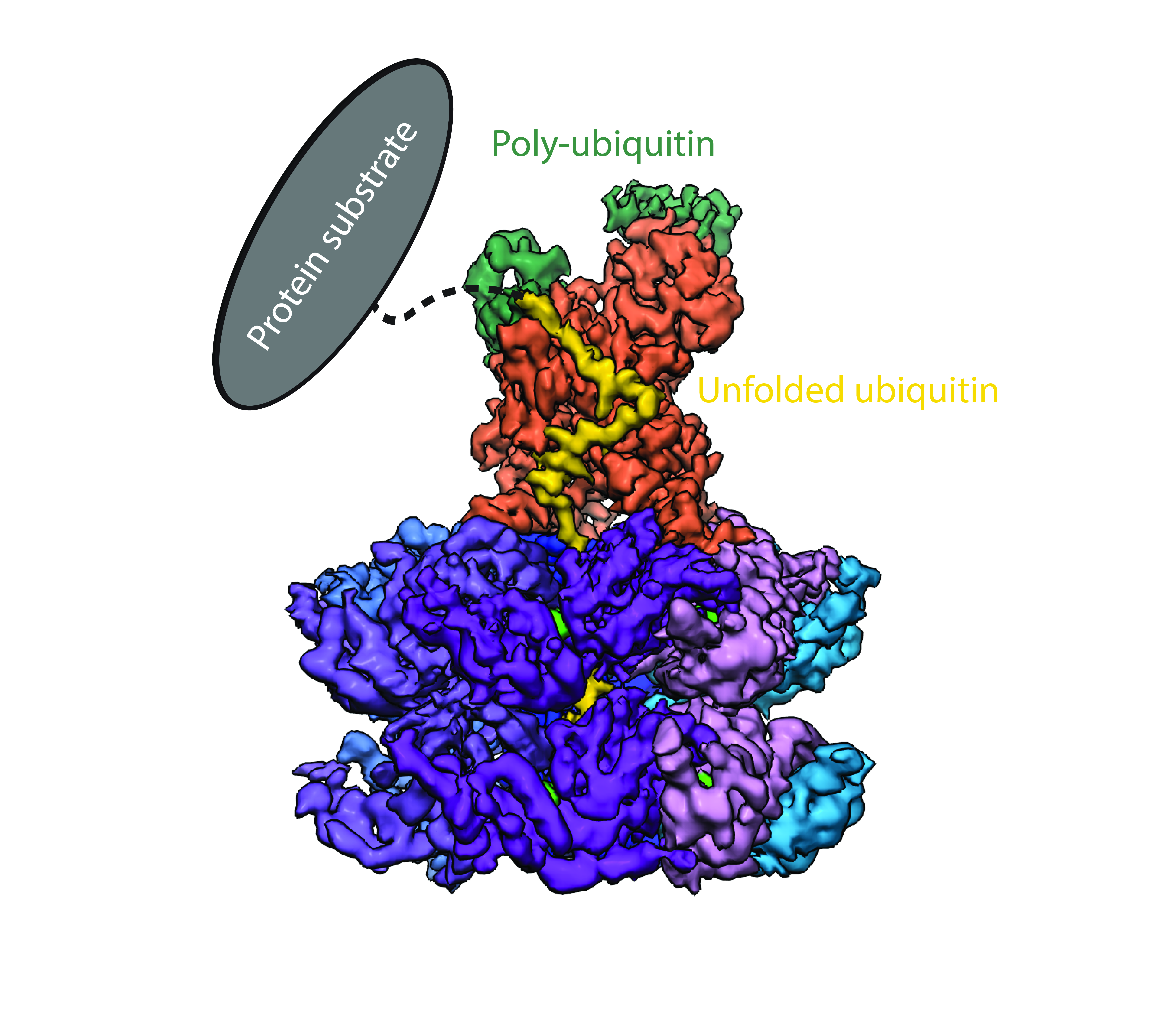
Proteins marked by poly-ubiquitin are degraded by the 26s proteasome. However, if proteins are too well-folded, in complexes, or located within membranes such as in the endoplasmic reticulum or mitochondria, these proteins cannot be directly degraded by the proteasome. To degrade these proteins, a protein called Cdc48 in yeast, named p97 or VCP in metazoans, must first unfold them upstream of the proteasome. Cdc48 is critical for cell viability, and mutations in p97 often lead to various neurodegenerative conditions in humans. In new findings published in Science, the Rapoport Lab used cryo-electron microscopy to understand the mechanism by which Cdc48 can interact with an unfold a wide variety of proteins. Cdc48 recruits the poly-ubiquitin signal, and initiates processing by unfolding a ubiquitin molecule, which it then uses as a handle to pull on, and unfold, attached protein substrates to prepare them for degradation by the proteasome.