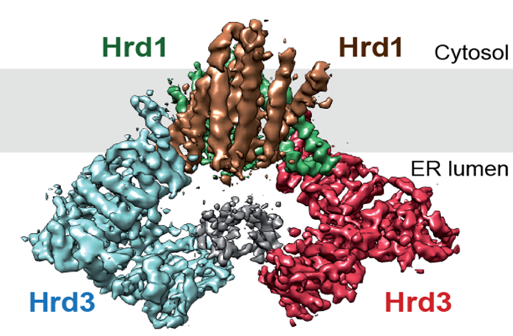
A conserved pathway called “endoplasmic reticulum associated protein degradation (ERAD) is responsible for the disposal of misfolded ER proteins. Previous work from the Rapoport lab indicated that the multi-spanning ubiquitin ligase Hrd1 is a key component of ERAD; Hrd1 allows misfolded luminal and membrane proteins to move from the ER into the cytosol. However, it remained unclear whether Hrd1 forms a protein-conducting channel. In a paper recently published in Nature, the Rapoport and Liao labs teamed up to determine a single particle cryo-EM structure of Hrd1 together with its luminal binding partner Hrd3. The Hrd1/Hrd3 complex structure at ~4 Å shows that Hrd1 forms a dimer inside the membrane with two Hrd3 molecules forming a luminal arch above the dimer. Each Hrd1 molecule has a large hydrophilic cavity extending from the cytosol almost to the ER lumen. A trans-membrane segment of the other Hrd1 molecule forms a lateral seal of the cavity. Both the cavity and the lateral gate are reminiscent of other protein-conducting conduits, such as the Sec61/SecY channel or the YidC protein, which allow proteins to move in the other direction, i.e. from the cytosol into the membrane. These results indicate that the thinning of the lipid bilayer may be a general principle employed by protein-conducting conduits to lower the energetic barrier for moving hydrophobic segments in or out of the membrane.