Most drugs are small molecules that cause a therapeutic effect by binding to a target protein. Some small molecules inhibit a protein’s function, whereas others work by activating the protein. In work published in Nature Chemical Biology, the King Lab reports the surprising identification of a small molecule that can do either, depending on cellular regulatory context.
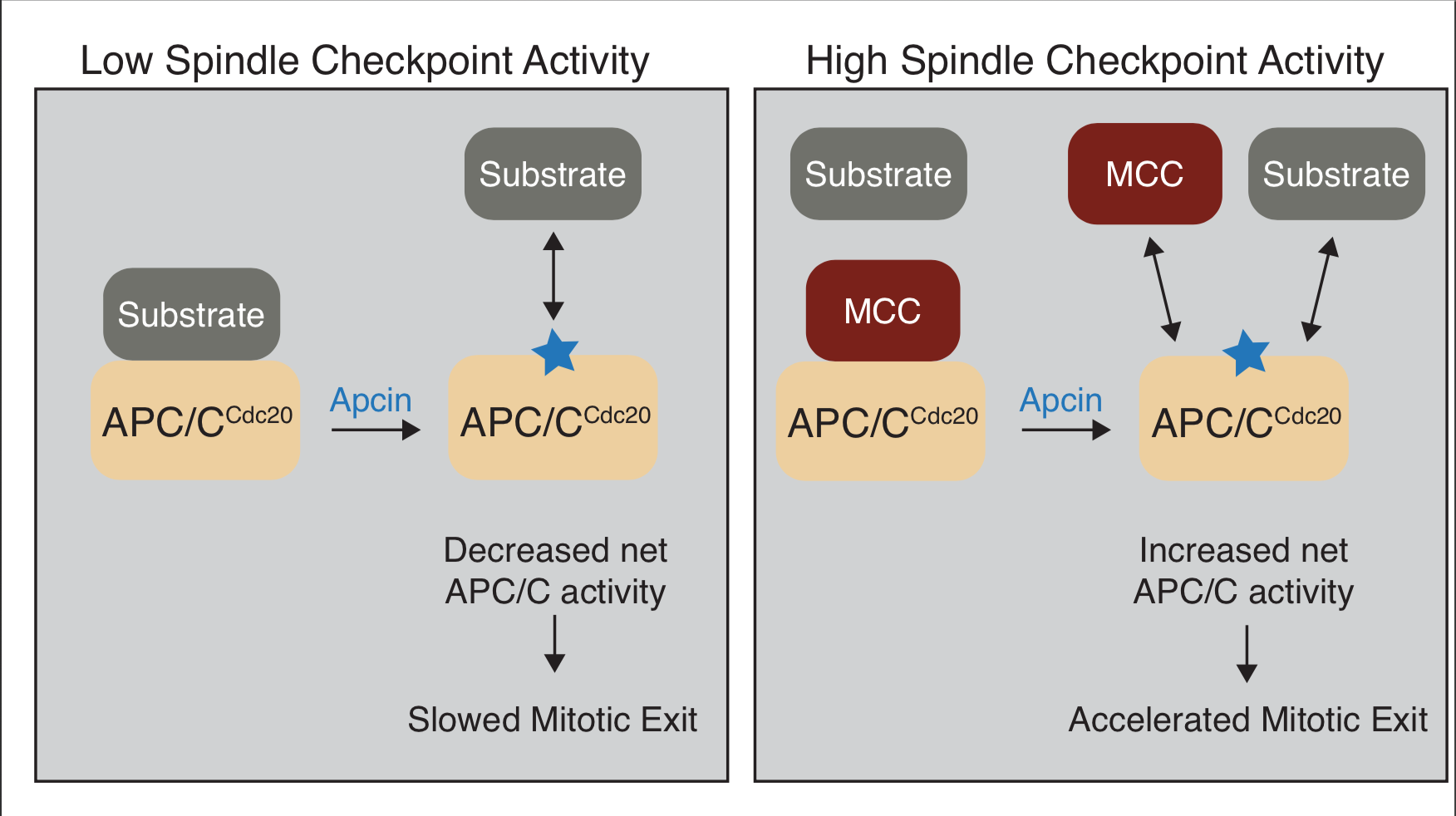
The King lab has pioneered the development of small molecule inhibitors of the Anaphase-Promoting Complex/Cyclosome (APC/C), a ubiquitin ligase that is required for anaphase and mitotic exit. In work led by graduate student Katie Richeson, the paper reports the surprising finding that the APC/C inhibitor apcin can paradoxically stimulate APC/C activity under conditions when the APC/C is antagonized the spindle checkpoint, a signaling pathway that normally restrains APC/C activity during mitosis. These findings indicate that apcin causes net inhibition of APC/C when spindle checkpoint activity is low, or net activation of APC/C when spindle checkpoint activity is high, indicating that apcin can act as either an inhibitor or an activator of APC/C depending on physiological context.