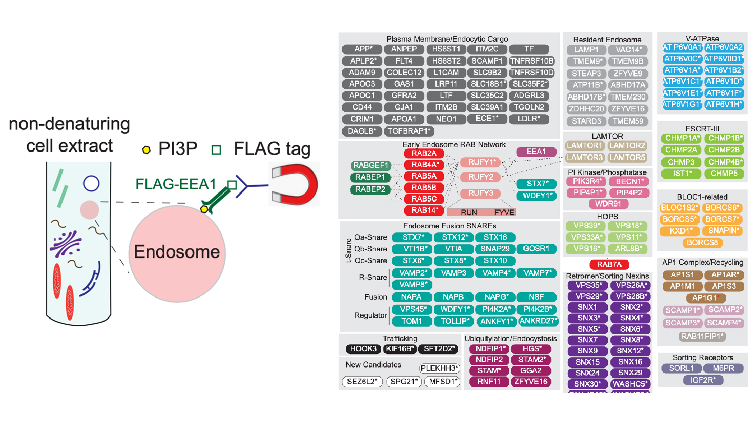
Understanding of the endolysosomal system has been significantly advanced through the development of methods for selective capture of lysosomes (for example, the Lyso-IP), but methods for analogous isolation of early intermediates in the endolysosomal system are lacking. In this study, Hankum Park, Frances Hundley, Qing Yu, Katherine Overmyer, Dain Brademan, Lia Serrano, Joao Paulo, Julia Paoli, Sharan Swarup, Joshua Coon, Steven Gygi, and Wade Harper developed a new, rapid method to isolate early/sorting endosomes via immunoprecipitation of affinity-tagged EEA1, termed Endo-IP. They found that Endo-IP efficiently and specifically enriches for endosomal proteins, including subunits of the HOPS complex, ESCRT-III, retromer, and AP1, as well as candidate cargo. Transferrin uptake assays and inhibition of endocytosis demonstrate that Endo-IP can detect dynamic endocytic events.
Among the endocytic cargo identified by Endo-IP was the amyloid precursor protein (APP) genetically linked with Alzheimer’s disease. Processing of APP to amyloidogenic Aβ peptides by β- and ?-Secretases can occur within the endolysosomal system, among other compartments, but methods for spatial quantification of Aβ products in individual organelles are lacking. The authors combined Endo- and Lyso-IP with targeted proteomics to provide a spatial digital snapshot of Aβ products. This analysis revealed that products of Aβ processing by β- and ?-Secretases, as well as alterations in the specificity of cleavage by small molecule ?-Secretase modulators, can be quantified in both early/sorting endosomes and lysosomes. The new Endo-IP approach will likely facilitate systematic interrogation of the many processes that are coordinated on early endosomes.