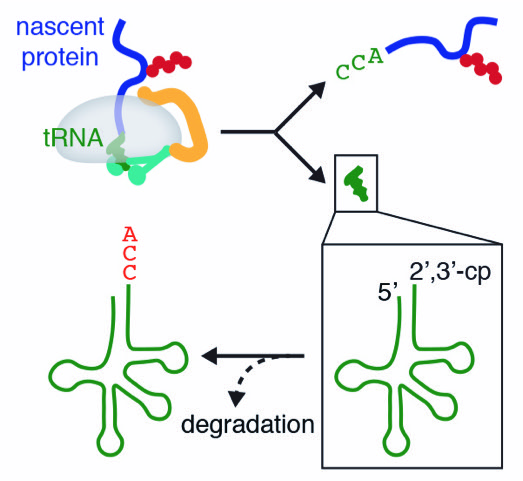
An inability to rescue ribosomes that stall during protein synthesis is associated with proteotoxicity and neurodegeneration. Stalled ribosomes harbor aberrant nascent proteins attached to tRNA. In their study inNSMB, the Shao Lab identified a mechanism that coordinates tRNA and protein quality control, in which a dedicated factor specifically cleaves off the invariant 3’-CCA from peptidyl-tRNA on stalled ribosomal complexes. This releases the nascent protein for degradation and produces a novel nonfunctional tRNA species that can be recycled by a two-step process with proofreading capabilities. These findings provide insights into how cells scrutinize individual components of aberrant molecular complexes to eliminate defective products without wasting functional factors.