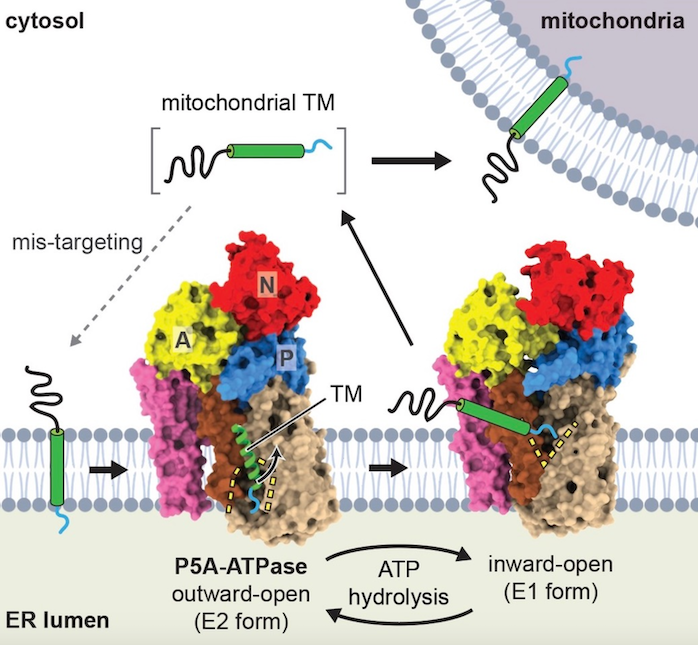
Eukaryotic cells contain different membrane-bound organelles whose identities and essential functions rely on protein composition. In a study published in Science, the Shao lab and the lab of former HMS Cell Biology student Eunyong Park (now at Berkeley) showed that the P5A-ATPase ATP13A1 (yeast Spf1) dislocates mis-inserted protein transmembrane segments from the endoplasmic reticulum (ER) to maintain organelle homeostasis. Functional work led by postdoctoral fellow Mike McKenna, together with CRSP student Jerry Wei and Alban Ordureau (Harper lab), identified that the P5A-ATPase directly interacts with and removes mislocalized mitochondrial proteins from the ER. Cryo-EM structures of the P5A-ATPase in different conformations determined by the Park lab revealed the molecular basis of substrate selection and dislocation. This work establishes a new class of substrates for this large family of transporters and identifies a previously unknown protein safeguarding mechanism at the ER.