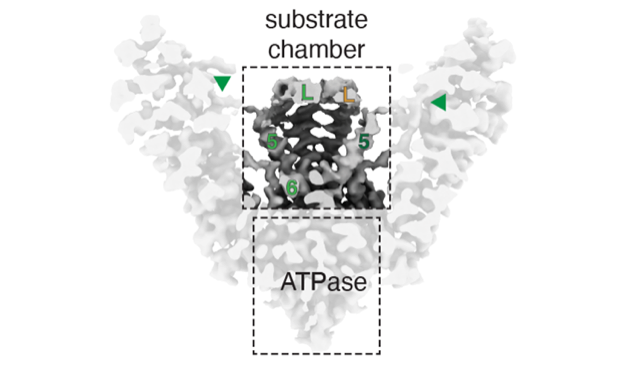
Close collaboration between chaperones is required during protein biosynthesis. The pre-targeting GET complex selectively triages nascent tail-anchored membrane proteins for biosynthesis at the endoplasmic reticulum (ER) through a coordinated transfer reaction between two chaperones which prevents off-target protein degradation. In a study in Nature Structural & Molecular Biology, the Shao lab determines cryo-EM structures of the metazoan pre-targeting GET complex. The structures show that GET3, the central protein chaperone and ATPase, is flanked by two arms comprised of accessory factors. The authors identify new interactions between GET3 and an accessory factor that remodel the substrate binding chamber and potentially coordinate protein loading with ATP hydrolysis to drive ER targeting. These findings reveal how accessory factors prime chaperones for cooperative and directional substrate transfer reactions during multi-step protein biosynthesis programs.