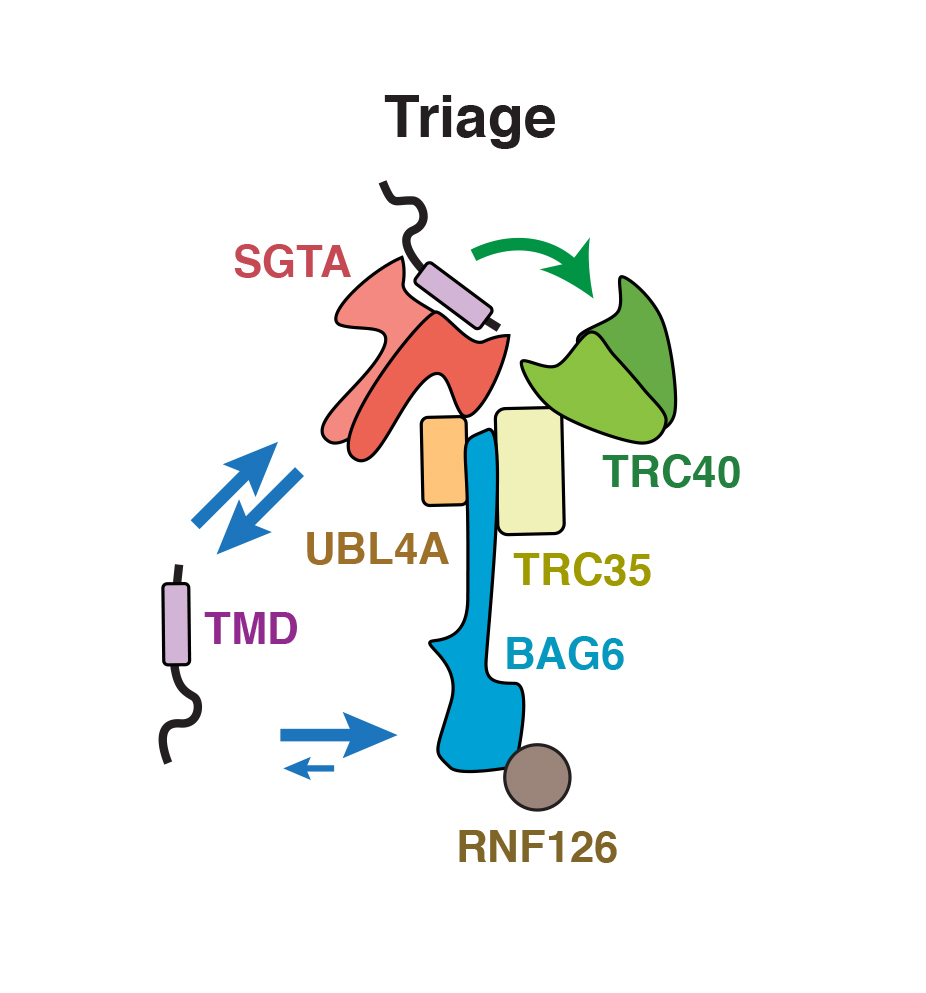
Protein biosynthesis and quality control must be precisely balanced to give new proteins an opportunity to mature before degrading failed intermediates that can cause disease. New work from Shao et al., published in Science, shows how a complex of three chaperones (TRC40, BAG6, and SGTA) triages a class of membrane proteins between biosynthesis and degradation. To do this, they biochemically rebuilt the triage reaction in a test tube with purified proteins and measured the kinetics of substrate flux through the complex. SGTA is the fastest at capturing new substrates to keep them uncommitted from any fate. Priority to biosynthesis is achieved by a ‘private’ and very fast substrate transfer reaction from SGTA to TRC40, which delivers substrates to the endoplasmic reticulum (ER). This transfer is analogous to a baton transfer between two runners in a relay race. Otherwise, BAG6 picks up substrates released from SGTA that have failed transfer to TRC40 and routes them for degradation. This is analogous to a relay race monitor removing a baton dropped because one of the runners was unavailable or out of place. Thus, how long SGTA can hold onto substrates (~20 sec) limits how long they have to mature. Because TRC40 and BAG6 hold onto substrates 15-30 times longer than SGTA, transfer to these chaperones effectively commits substrates to ER targeting or quality control, respectively. This work introduces molecular concepts that are generally applicable to numerous protein triage processes that are crucial for preventing the accumulation of faulty protein by-products linked to various neurodegenerative and aging diseases.