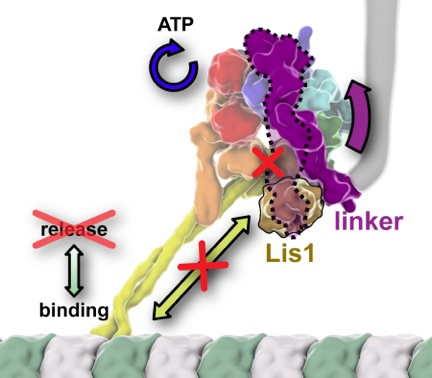
 Cytoskeletal molecular motors move uni-directionally along their tracks. This poses multiple problems: How do they get to the start of the track? Once there, how do they stay there to capture cargo? A candidate for retaining the microtubule-based motor dynein at the start of its track (microtubule plus ends) is a ubiquitous regulator called Lis1. The Reck-Peterson Lab, in collaboration with the lab of Andres Leschziner (Harvard) has been seeking to answer the second question. Previously they showed that Lis1, a gene mutated in the rare neurodevelopmental disease lissencephaly, had the functional properties necessary to stall dynein on microtubules: when bound to Lis1, dynein keeps going through the ATP hydrolysis cycle that normally powers the motor yet, rather than walking, it remains tightly bound to its track. This led to the proposal that Lis1 was working as a “clutch”, uncoupling dynein’s engine (the ATP hydrolysis cycle) from its wheels (the cycle of microtubule binding and release). In the latest issue of eLife, the Reck-Peterson and Leschziner groups now shed light on the molecular mechanism of Lis1’s affect on dynein. They reported three distinct 3D cryo-electron microscopy structures of the dynein/Lis1 complex, representing the first 3D structures of dynein bound to any of its regulators. Their structures revealed that Lis1 binding to dynein causes a repositioning of dynein’s main mechanical element, the “linker”. Based on these structures they hypothesized that Lis1 physically blocks an interaction between dynein’s linker and AAA+ motor ring, known to be required for dynein to release from microtubules. Förster resonance energy transfer (FRET), single-molecule and in vivo assays supported this hypothesis. Strikingly, shortening dynein’s linker to a point where it could physically bypass Lis1 made dynein Lis1-insensitive, showing that Lis1 keeps dynein in a persistent microtubule-bound state by directly blocking progression of its mechanochemical cycle.
Cytoskeletal molecular motors move uni-directionally along their tracks. This poses multiple problems: How do they get to the start of the track? Once there, how do they stay there to capture cargo? A candidate for retaining the microtubule-based motor dynein at the start of its track (microtubule plus ends) is a ubiquitous regulator called Lis1. The Reck-Peterson Lab, in collaboration with the lab of Andres Leschziner (Harvard) has been seeking to answer the second question. Previously they showed that Lis1, a gene mutated in the rare neurodevelopmental disease lissencephaly, had the functional properties necessary to stall dynein on microtubules: when bound to Lis1, dynein keeps going through the ATP hydrolysis cycle that normally powers the motor yet, rather than walking, it remains tightly bound to its track. This led to the proposal that Lis1 was working as a “clutch”, uncoupling dynein’s engine (the ATP hydrolysis cycle) from its wheels (the cycle of microtubule binding and release). In the latest issue of eLife, the Reck-Peterson and Leschziner groups now shed light on the molecular mechanism of Lis1’s affect on dynein. They reported three distinct 3D cryo-electron microscopy structures of the dynein/Lis1 complex, representing the first 3D structures of dynein bound to any of its regulators. Their structures revealed that Lis1 binding to dynein causes a repositioning of dynein’s main mechanical element, the “linker”. Based on these structures they hypothesized that Lis1 physically blocks an interaction between dynein’s linker and AAA+ motor ring, known to be required for dynein to release from microtubules. Förster resonance energy transfer (FRET), single-molecule and in vivo assays supported this hypothesis. Strikingly, shortening dynein’s linker to a point where it could physically bypass Lis1 made dynein Lis1-insensitive, showing that Lis1 keeps dynein in a persistent microtubule-bound state by directly blocking progression of its mechanochemical cycle.
For more details, click here.