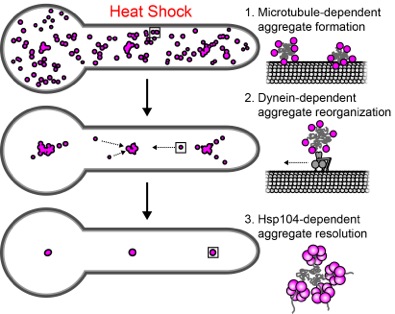
 Organisms across the evolutionary spectrum have evolved mechanisms to maintain the integrity of the cellular proteome. Among these mechanisms are spatial protein quality control pathways in which damaged and misfolded cellular proteins are actively sequestered at unique subcellular structures in response to acute stress. This mitigates the deleterious effects of these aberrant protein species, which can include advanced cellular aging and cytotoxicity leading to cell death. Despite the universal importance of such spatial control of the proteome, there is considerable mechanistic diversity throughout the evolutionary scale regarding how this control is achieved. In a recent publication in Cell Reports(Egan and McClintock et al., PMID 25865884) the Reck-Peterson Lab expanded on the known evolutionary diversity of spatial quality control mechanisms by examining the subcellular organization of heat-induced protein aggregates in filamentous fungi, which are of substantial health and economic importance and serve as a model for transport processes in other polarized eukaryotic cells. Using Aspergillus nidulans, the Reck-Peterson group found that protein aggregates are actively organized at periodic subcellular structures in a process dependent on microtubules and their associated motor dynein. In addition, they found that sustained stress and increased burdening of this spatial quality control pathway can lead to defects in other microtubule-based transport processes. Given the significance of protein aggregation and polarized transport in neurodegenerative disorders, as well as the pathogenicity of many filamentous fungi, this work suggests several avenues of further investigation for understanding and combating disease.
Organisms across the evolutionary spectrum have evolved mechanisms to maintain the integrity of the cellular proteome. Among these mechanisms are spatial protein quality control pathways in which damaged and misfolded cellular proteins are actively sequestered at unique subcellular structures in response to acute stress. This mitigates the deleterious effects of these aberrant protein species, which can include advanced cellular aging and cytotoxicity leading to cell death. Despite the universal importance of such spatial control of the proteome, there is considerable mechanistic diversity throughout the evolutionary scale regarding how this control is achieved. In a recent publication in Cell Reports(Egan and McClintock et al., PMID 25865884) the Reck-Peterson Lab expanded on the known evolutionary diversity of spatial quality control mechanisms by examining the subcellular organization of heat-induced protein aggregates in filamentous fungi, which are of substantial health and economic importance and serve as a model for transport processes in other polarized eukaryotic cells. Using Aspergillus nidulans, the Reck-Peterson group found that protein aggregates are actively organized at periodic subcellular structures in a process dependent on microtubules and their associated motor dynein. In addition, they found that sustained stress and increased burdening of this spatial quality control pathway can lead to defects in other microtubule-based transport processes. Given the significance of protein aggregation and polarized transport in neurodegenerative disorders, as well as the pathogenicity of many filamentous fungi, this work suggests several avenues of further investigation for understanding and combating disease.