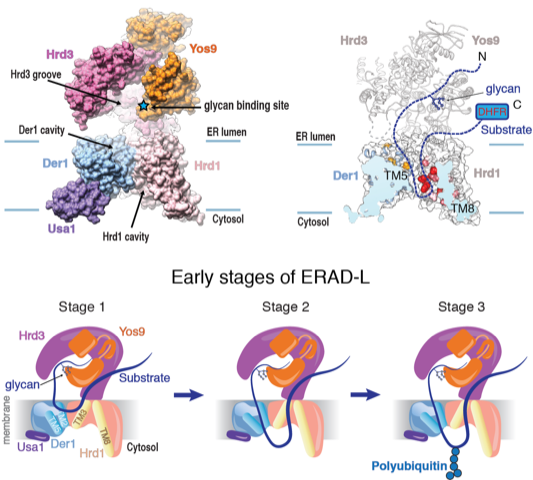
ER-associated protein degradation (ERAD) disposes of misfolded endoplasmic reticulum (ER) proteins. ERAD also mediates the regulated degradation of folded ER proteins and is hijacked by certain viruses. Misfolded luminal ER proteins undergo ERAD-L: they are retrotranslocated into the cytosol, polyubiquitinated, and degraded by the proteasome. ERAD-L is mediated by the Hrd1 complex, a complex of five proteins, but the mechanism of retrotranslocation has remained mysterious. In new findings published in Science, the Rapoport Lab, with the help of the lab of Maofu Liao, used cryo-electron microscopy to determine the architecture of the entire active Hrd1 complex. These structures, along with crosslinking and molecular dynamics simulation results, suggest how the Hrd1 complex recruits and retrotranslocates its substrates. The study shows that the Hrd1 complex retrotranslocates misfolded luminal ER proteins through two “half-channels” juxtaposed in a thinned membrane. This arrangement lowers the energy barrier for a polypeptide loop to pass through the membrane. The demonstrated novel mechanism may also be found in other translocation systems.