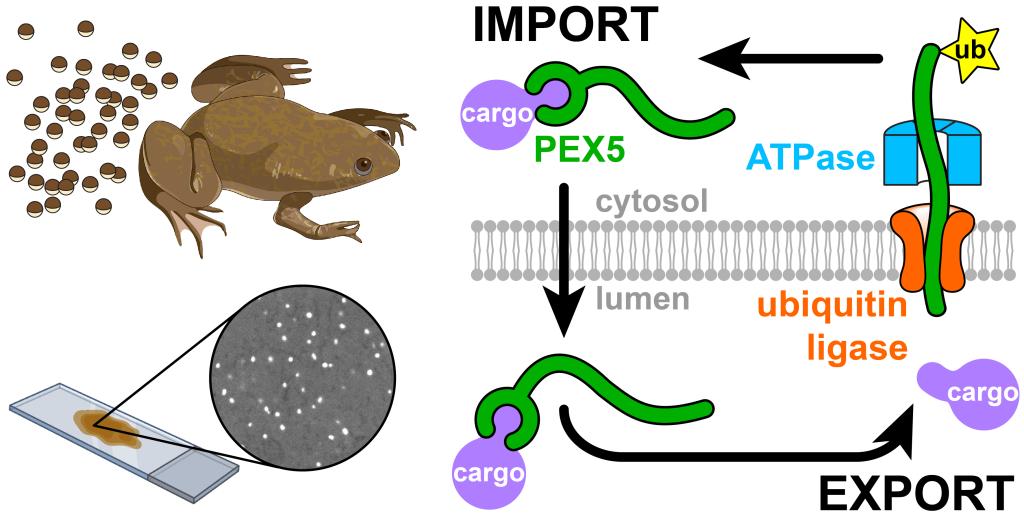
Peroxisomes are organelles that are vital for human health, as they perform important functions related to lipid metabolism, redox homeostasis, and the synthesis of myelin. The absence of functional peroxisomes causes life-threatening neurological disorders, many of which arise from defective import of enzymes into the organelle. Peroxisomal enzymes are made in the cytosol, and brought to peroxisomes by a soluble receptor called PEX5. PEX5 then moves the cargo into the lumen, but the underlying mechanism has been elusive.
In this study, Michael Skowyra and Tom Rapoport use a novel cell-free system based on frog egg extract to investigate how PEX5 imports proteins into peroxisomes. They show that PEX5 shuttles proteins across the peroxisomal membrane completely into the lumen of the organelle. PEX5 is then exported out of the lumen back into the cytosol. To trigger export, PEX5 inserts a flexible segment into a membrane-embedded ubiquitin ligase complex. The ligase then attaches a single ubiquitin to a conserved cysteine in PEX5, which allows PEX5 to be pulled out from the lumen by an ATPase motor. PEX5 is unfolded during the export step, causing bound cargo to be stripped off and left behind in the lumen. PEX5 can then begin another round of import after presumably refolding in the cytosol. This work thus reveals how PEX5 cycles between the cytosol and peroxisomes to import proteins into the organelle.
The full paper is available here.