The endoplasmic reticulum (ER) is shaped by curvature-generating proteins, including the reticulons (Rtns) and a subfamily of the REEPs (REEP5,6 in mammals and Yop1 in yeast). The Rtns and REEP5 subfamily have redundant functions in generating the high membrane-curvature characteristic of the tubular ER and sheet edges. The REEPs contain a second subfamily, which in mammals includes REEP1-4. The function of this REEP1 subfamily is unknown.
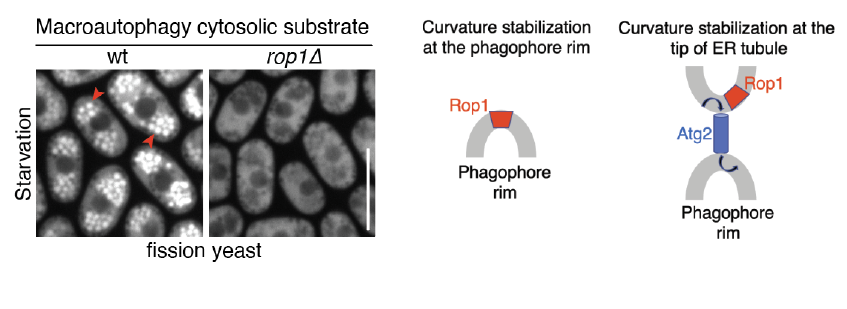
In this study, Ning Wang, Yoko Shibata, Joao Paulo, Steven Gygi and Tom Rapoport show that Rop1, the single member of the REEP1 subfamily in the fission yeast Schizosacharomyces pombe, is crucial for macroautophagy (autophagy) of organelles and cytosolic proteins. They show that Rop1 is needed for the formation of phagophores, the cup-like structures consisting of two closely apposed membrane sheets that encapsulate cargo. Rop1 is recruited at early stages to phagophores and is required for their maturation into autophagosomes. Rop1 function relies on its ability to generate high membrane curvature and on its colocalization with the autophagy component Atg2 that is thought to reside at the phagophore rim. It is proposed that Rop1 facilitates the formation and growth of the double-membrane structure of the autophagosome by two non-exclusive models: 1) it resides on the phagophore rim to stabilize the high membrane-curvature; 2) it stabilizes the curvature at the tip of ER tubule to support lipid transfer from the ER to phagophores.