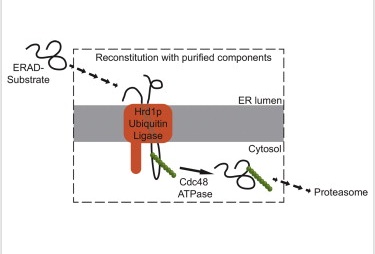
 Proteins that are translocated into the endoplasmic reticulum (ER) undergo quality control so that only correctly folded proteins are moved on in the secretory pathway. If a protein cannot reach its native folded state, it is ultimately transported back into the cytosol, poly-ubiquitinated, and degraded by the proteasome, a process called ER-associated protein degradation (ERAD). How proteins are retro-translocated across the ER membrane and moved into the cytosol is only poorly understood. Previous work demonstrated that the ubiquitin ligase Hrd1p is the only membrane component needed for a basic ERAD process. Now, the Rapoport group shows that key steps of basic ERAD can be recapitulated with purified components (Stein et al., Cell 158, 1375-1388). Hrd1p binds misfolded protein substrates, discriminating them from folded proteins. Subsequently, both Hrd1p and substrate are poly-ubiquitinated. Next, the Cdc48p ATPase complex binds and uses the energy of ATP hydrolysis to release substrate from Hrd1p. Finally, ubiquitin chains are trimmed by the de-ubiquitinating enzyme Otu1p, which is recruited and activated by the Cdc48p complex. The Cdc48-dependent extraction of poly-ubiquitinated proteins from the membrane can be reproduced with reconstituted proteoliposomes. These results provide a major step forward towards the goal to recapitulate ERAD with purified components and to elucidate the molecular mechanism of the process.
Proteins that are translocated into the endoplasmic reticulum (ER) undergo quality control so that only correctly folded proteins are moved on in the secretory pathway. If a protein cannot reach its native folded state, it is ultimately transported back into the cytosol, poly-ubiquitinated, and degraded by the proteasome, a process called ER-associated protein degradation (ERAD). How proteins are retro-translocated across the ER membrane and moved into the cytosol is only poorly understood. Previous work demonstrated that the ubiquitin ligase Hrd1p is the only membrane component needed for a basic ERAD process. Now, the Rapoport group shows that key steps of basic ERAD can be recapitulated with purified components (Stein et al., Cell 158, 1375-1388). Hrd1p binds misfolded protein substrates, discriminating them from folded proteins. Subsequently, both Hrd1p and substrate are poly-ubiquitinated. Next, the Cdc48p ATPase complex binds and uses the energy of ATP hydrolysis to release substrate from Hrd1p. Finally, ubiquitin chains are trimmed by the de-ubiquitinating enzyme Otu1p, which is recruited and activated by the Cdc48p complex. The Cdc48-dependent extraction of poly-ubiquitinated proteins from the membrane can be reproduced with reconstituted proteoliposomes. These results provide a major step forward towards the goal to recapitulate ERAD with purified components and to elucidate the molecular mechanism of the process.