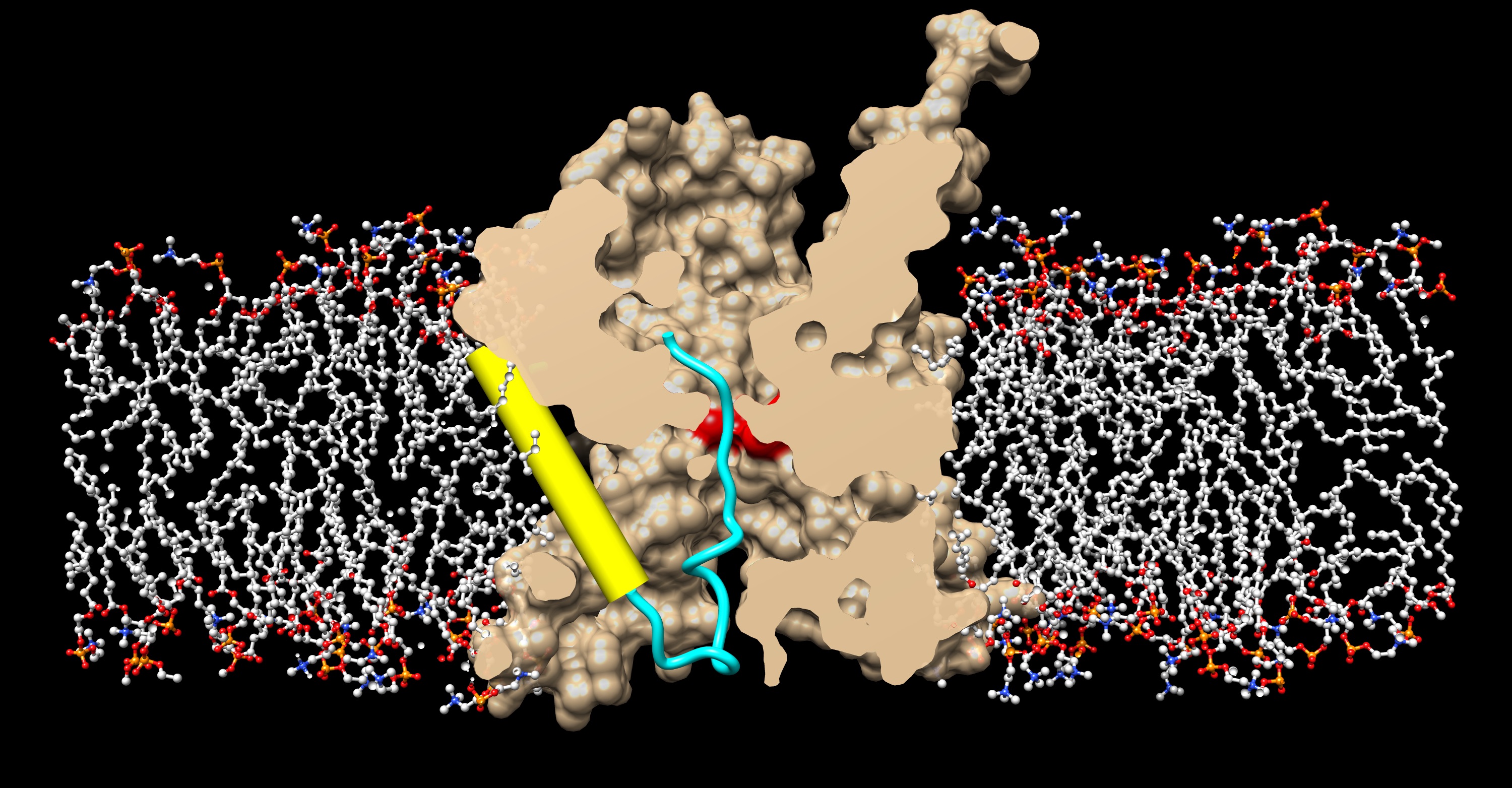
A recent paper by Li et al. (Rapoport lab, with help from Hidde Ploegh’s lab at the MIT), reports a crystal structure of the active protein translocation channel, which had been a “holy grail” in the field. Previously, the Rapoport lab had reported crystal structures of the idle channel (van den Berg, B.et al. Nature 427, 36-44 (2004)) and of a complex of the channel with the translocation ATPase SecA (Zimmer et al. Nature 455, 936-943 (2008)). Structures of the idle channel show that the largest subunit, SecY, consists of two halves that form an hourglass-shaped pore and a lateral gate that faces lipid. The constriction in the middle of the membrane is formed from six pore ring residues. The cytoplasmic funnel is empty, while the extracellular funnel is filled with a plug domain. When SecA binds to the channel, it inserts a two-helix finger into the cytoplasmic opening of the channel and partially opens the lateral gate. The new structure now contains the SecY channel, SecA, and a short segment of a secretory protein fused into the two-helix finger of SecA. This segment inserts into the channel as a loop, displacing the plug domain. The hydrophobic core of the signal sequence forms a helix that sits in a groove outside the lateral gate, while the following polypeptide segment intercalates into the gate. The C-terminal section of the polypeptide loop is located in the channel, surrounded by four pore ring residues. The structure shows that the hydrophobic regions of signal sequences exit the lateral gate and partition into the lipid phase, explaining their diversity in sequence and length. A comparison with other structures shows that this mechanism of signal sequence recognition is universal, regardless of the organism and mode of translocation, and also applies to bilayer-spanning domains of nascent membrane proteins.