How the shape of an organelle is generated is only poorly understood, but is a fundamental question in cell biology. An interesting example is the endoplasmic reticulum (ER) as it consists of morphologically distinct domains. The ER comprises the nuclear envelope and the peripheral ER that consists of tubules connected by three-way junctions into a network, as well as interdispersed sheets. During mitosis, the tubular ER network converts into sheets. Two protein families, the reticulons (Rtns) and DP1 stabilize the high membrane curvature of tubules in cross-section. Fusion is mediated by membrane-bound GTPases of the dynamin family, called atlastins (ATLs) in metazoans. A third protein, termed lunapark, is reported to be involved in ER morphology and its exact role is unknown. Work from the Rapoport Lab, published in eLife, has elucidated the interrelationship between ATL, Rtns/DP1, and Lnp, using mammalian cells and frog egg extracts. Surprisingly, ATL is not only required to form an ER network, but also to maintain it. A balance between ATL and Rtn activity is needed for network maintenance; high concentrations of Rtn disassemble the ER into vesicles, but this can be reversed by increasing the concentration of ATL. The results suggest a model in which ATL tethers and fuses tubules stabilized by Rtns. Lnp subsequently moves into three-way junctions and probably stabilizes them. Loss-of-function of Lnp in both mammalian cells and frog egg extracts leads to the expansion of peripheral sheets. During mitosis, Lnp is phosphorylated and inactivated, suggesting that Lnp may contribute to the characteristic tubule-to-sheet transition of the ER.
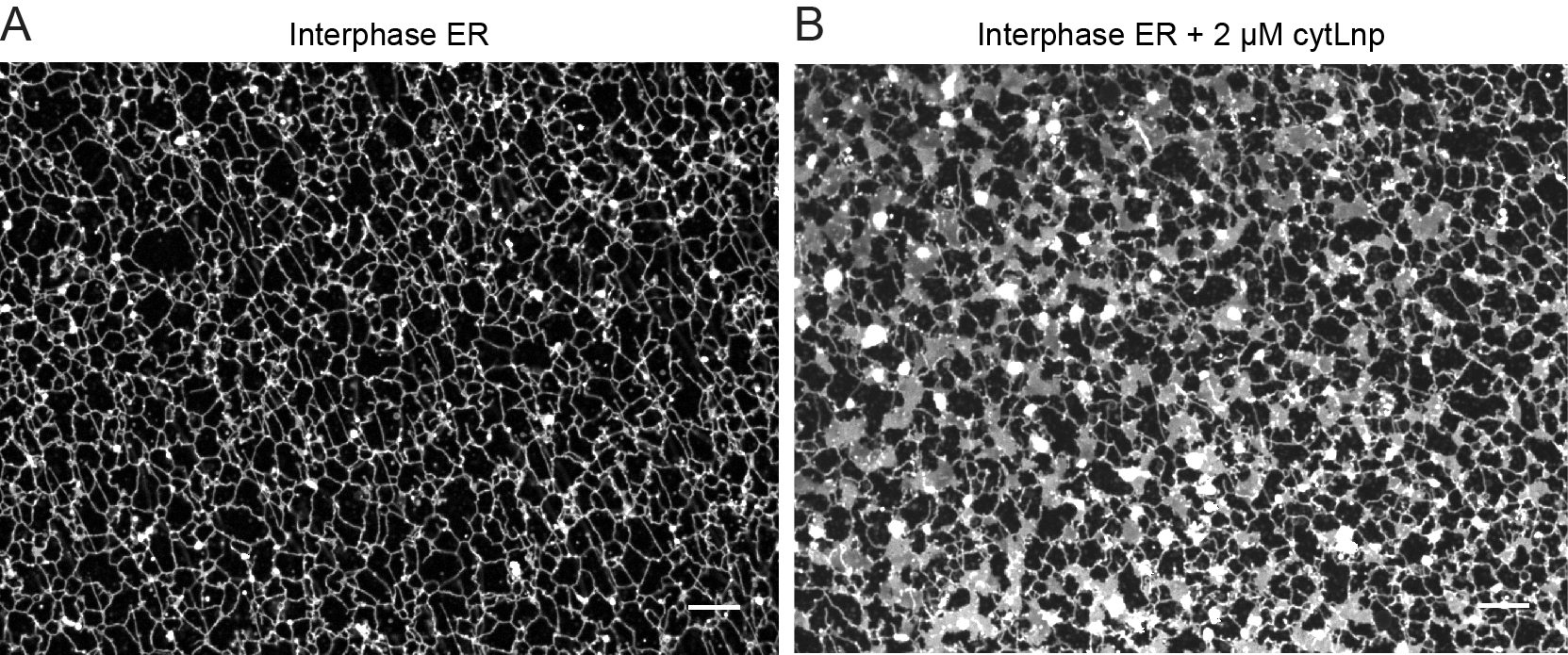
(A) A tubular ER network was assembled from the interphase frog egg extracts.
(B) The addition of a cytosolic fragment of Lnp into the frog egg extracts converted the tubular ER network into sheets.