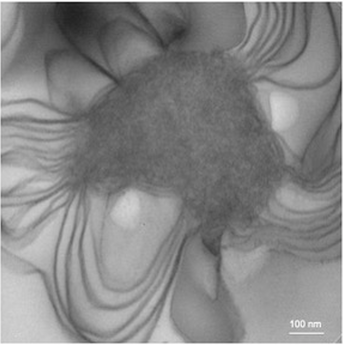
Pulmonary surfactant forms a thin extracellular layer that drastically reduces surface tension and is thus critical for breathing. Surfactant is initially stored in lamellar bodies (LBs) as concentric stacks of membrane layers. LB formation depends on surfactant protein B (SP-B), which is synthesized as a precursor (pre-proSP-B) and cleaved during intra-cellular trafficking into three related proteins (SP-BN, SP-BM, and SP-BC). In a recent paper in Mol. Cell, Sever et al. analyze the role of the three SP-B proteins in LB formation. Crystal structures and biochemical assays show that SP-BN is a non-specific phospholipid -binding and -transfer protein. Lipid binding to the SP-BN domain is required for vesicular export of proSP-B from the ER and neonatal viability in mice. ER export of proSP-B is also facilitated by the SP-BC domain. Once proSP-B arrives in a low-pH compartment, lipid transfer activity of the SP-BN domain is activated, resulting in the formation of lipoprotein particles and sorting of proSP-B to LBs. SP-BN is an unusual lipid transfer protein, as it moves phospholipids from a bilayer to a protein domain (SP-BM), rather than to another bilayer. Following proteolytic cleavage of proSP-B, the mature SP-BM protein generates the striking membrane layers characteristic of LBs. SP-BM alone, a protein containing only 79 amino acids, is sufficient to form these structures (see image). The only other organelle that has been reconstituted with purified proteins is the tubular ER network. Taken together, these results show how the three related SP-B domains cooperate in LB formation.