Exposure to environmental cold temperatures causes energy and metabolic adjustments necessary for cellular fitness and adaptation across tissues. Mammals are homeotherms and require active brown fat respiratory/thermogenic function to maintain whole-body temperature in response to cold weather. Cold-induced brown fat activation is supported by high increases in complete oxidation of glucose, fatty acids, and branched-chain amino acids that generate heat and maintain body temperature. Brown fat high oxidative power capacity relies on an extensive network of mitochondria with densely packed cristae harboring the respiratory complexes that are required for full nutrient oxidation and heat generation. In humans exposed to cold, rates of glucose uptake in brown fat are increased by nine times, depicting the high capacity of fuel utilization in these cells. However, the molecular and structural mechanisms in respiratory complexes that catalyze electron transfer and allow such an increase in nutrient oxidative power to heat the whole-body during cold adaptation remain elusive.
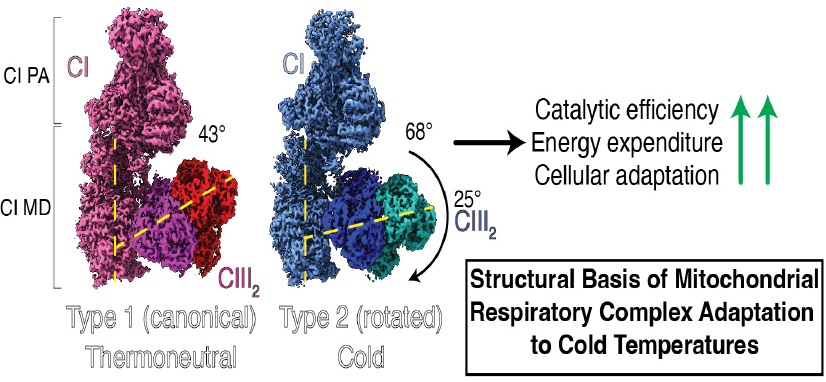
In this work, collaborative efforts from the Puigserver (DFCI/HMS), Liao (HMS and now SUSTech University), Sharma (University of Helsinki), and Gygi (HMS) laboratories combined thermoregulatory physiology, genetic mouse models, cryo-EM and hybrid quantum mechanical and molecular dynamics simulations to understand the mechanisms of cellular respiratory adaptation to lower environmental temperatures. They found that cold-activated and highly thermogenic brown fat re-structures the architecture of respiratory complexes I:III2 (CI:III2), where CIII2 rotates 25° relative to CI. This structural conformation is uniquely present in cold-acclimated but not in thermoneutral or in cold-acclimated ER stress PERK-/- mice, which exhibit severe defective whole-body temperature regulation and respiratory function. Unlike canonical (or type 1) CI:III2 assemblies, rotated (or type 2) complexes display features of high catalytic efficiency and electron transfer that facilitate nutrient oxidation and heat production in mitochondria. Mechanistically, cold-induced ER stress PERK-derived phospholipids remodel the mitochondrial inner membrane lipidome and allow the transition from type 1 to type 2 respiratory complexes. These results show unprecedented evidence of the plasticity of respiratory complexes to adjust their architecture to satisfy cellular bioenergetics under cold temperatures. These studies have implications in the understanding of the mechanisms of cellular respiration under cold stress and might also apply to other tissues with high energetic demands, including exercised cardiac or skeletal muscle and brain.