While targeted drug therapy at the oncogene BRAF(V600E) improves overall survival for melanoma patients, its potent therapeutic effect is heavily dampened by the development of BRAF inhibitor resistance.
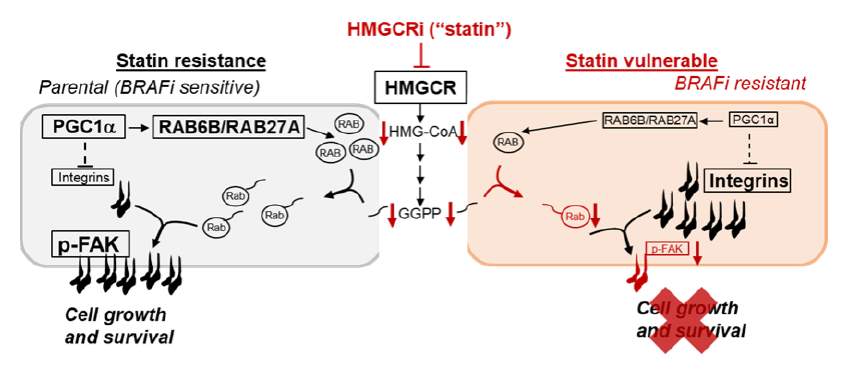
In their recent work published in Nature Communications, the Puigserver Lab found that epigenetic suppression of the mitochondrial biogenesis co-regulator PGC1α defines a unique and highly aggressive subset BRAF resistant melanomas. Through a metabolism-centered pharmacological screen, they identify HMGCR inhibitors (statins) that specifically restrain this subtype of aggressive BRAF resistant melanoma. PGC1-α suppression drives transcriptional downregulation of RAB6B and RAB27A levels, whereby their combined ectopic expression is sufficient to reverse HMGCR inhibitor sensitivity both in vitro and in vivo. Because integrin and FAK signaling is elevated in BRAF-inhibitor resistant cells with reduced PGC1α expression, these cells are endowed to survive in the absence of attachment. However, statin treatment in these resistant cells induces cell death through lowering membrane retention of RAB6B/RAB27A, which depletes plasma membrane integrin complexes and reduces downstream FAK activity. These studies suggest that chronic adaptation to oncogene-targeted treatments drive novel collateral metabolic vulnerabilities, and that HMGCR inhibitors could offer a combinatorial strategy to treat subsets of resistant melanomas.