Puigserver Lab identifies a chaperone-assisted mechanism controlling protein insertion into mitochondria
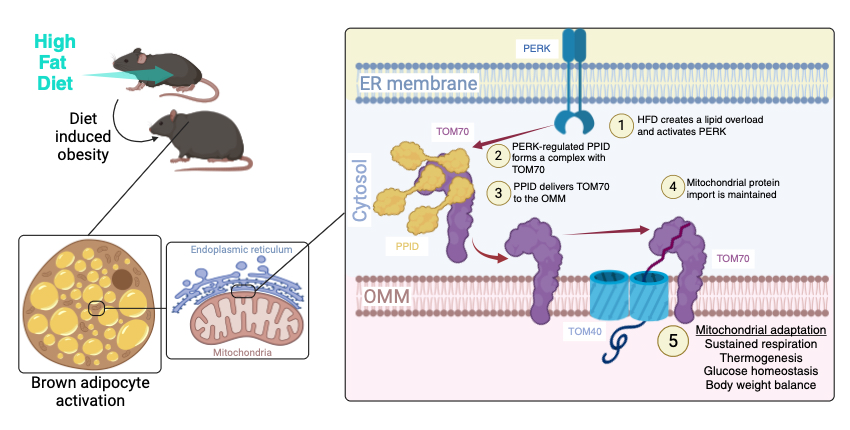
Obesity is a highly prevalent preclinical condition and a risk factor for several serious diseases such as type 2 diabetes (T2D), cardiovascular disease, or cancer. Increasing energy expenditure in thermogenic brown or brown-like adipose tissue (BAT) can counteract obesity and associated diseases. BAT metabolic functions rely on the crosstalk between an extensive mitochondrial network and other organelles such as the endoplasmic reticulum (ER) that create a unique landscape of protein-protein interactions, signaling and trafficking pathways to maintain adaptive cellular functions during diet-induced stress. At the Outer Mitochondrial Membrane (OMM), protein complexes, including the mitochondrial protein and lipid import machineries are fundamental to maintaining the mitochondrial function. However, the molecular mechanisms of interorganelle communication, especially the components that signal to and regulate the OMM, that sustain cellular energy metabolism during HFD stress in obesity and T2D are largely unknown.
In this work, collaborative efforts from the Puigserver (DFCI/HMS), Eck (DFCI/HMS), Kajimura (BIDMC), and Gygi (HMS) laboratories found that PPID (peptidyl-prolyl isomerase D/cyclophilin 40/Cyp40) is a PERK-activated cytosolic chaperone that drives the insertion of the mitochondrial import receptor TOM70 at the OMM. In vivo PPID suppression exacerbates obese and T2D phenotypes which correlate with defective respiratory/thermogenic function in murine brown adipocytes. Mechanistically, PPID C-terminal tetratricopeptide repeats (TPR), show specificity towards TOM70 core and C-tail domains, and facilitate OMM insertion, in a process that requires proline isomerization of the substrate TOM70. These results suggest a role for ER-stress-activated chaperones in controlling energy metabolism through a selective OMM protein insertion mechanism with implications in adaptation to cold temperatures and high-calorie diets.