Traditionally, it was assumed that cancer genomes evolve by accruing small-scale changes gradually, over many generations. However, the extreme complexity of many cancer genomes has led to an alternative view that they can evolve rapidly via discrete episodes that generate bursts of genomic alterations. One recently discovered catastrophic mutational processis called chromothripsis, which involves massive rearrangement of only one or a few chromosomes, generating an unusual DNA copy number pattern. The mechanism for chromothripsis had not been known, but prior work from the Pellman lab established that it can originate from abnormal nuclear structures, common in cancer, called micronuclei. Micronuclei have fragile nuclear envelopes (NE, work from the Hetzer laboratory, Salk Institute) whose spontaneous “rupture” somehow leads to chromosome fragmentation.
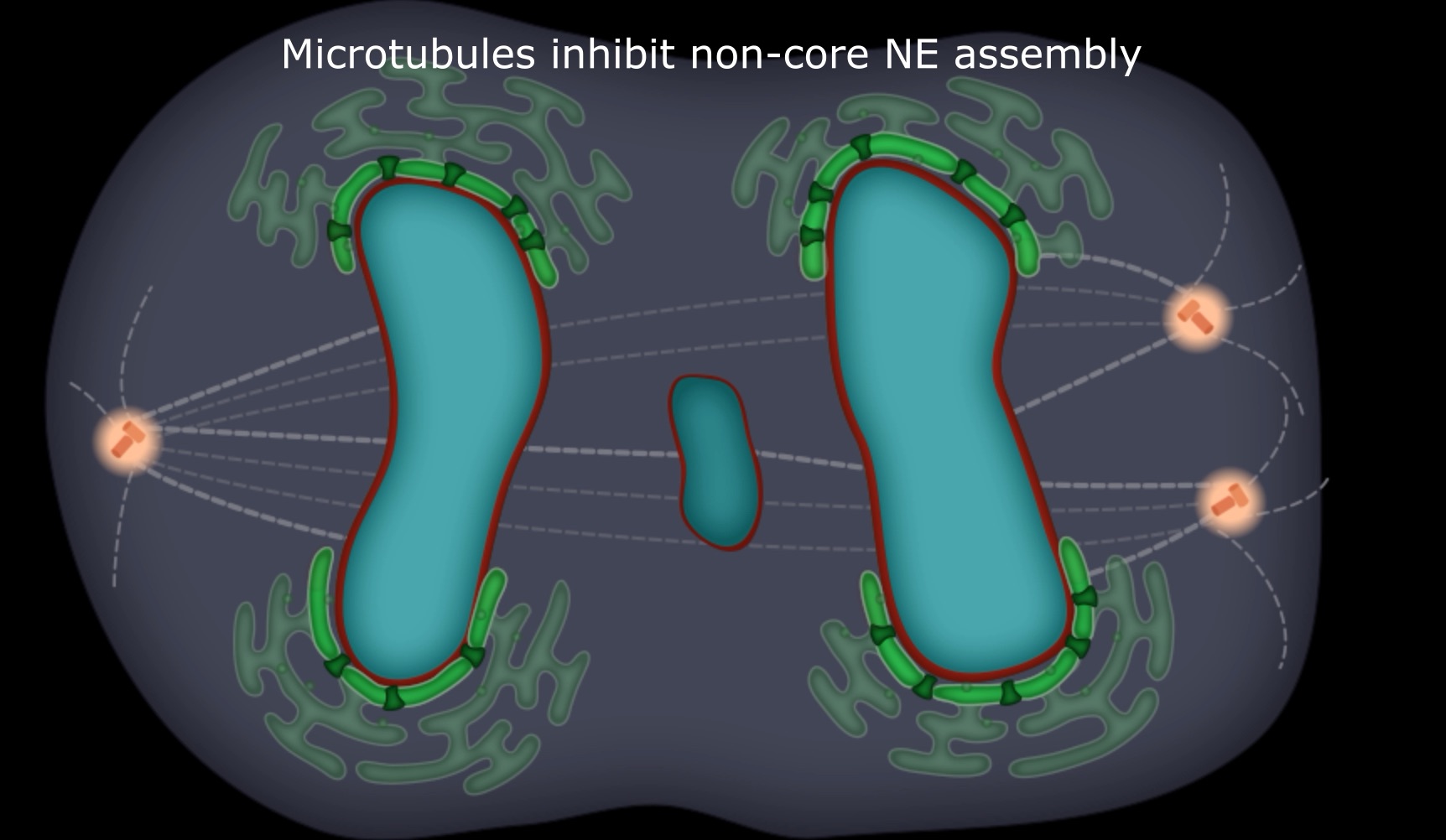
In the current paper in Nature, Shiwei Liu, Mijung Kwon and colleagues addressed the basis for NE fragility in micronuclei. They found that only a subset of NE proteins properly assemble on missegregated chromsoomes that “lag” within the mitotic spindle and then form micronuclei. The missing proteins include the nuclear pore complexes, producing nuclear transport defects in micronuclei. This results in the failure of these structures to normally accumulate key proteins involved in NE integrity and genome maintenance. In contrast to a previously proposed checkpoint model, the current data indicate that what inhibits the assembly of nuclear pore complexes on lagging chromosomes is the spindle microtubules themselves. Accordingly, experimental manipulations that position missegregated chromosomes away from the spindle correct defective nuclear envelope assembly, prevent spontaneous nuclear envelope disruption, and suppress DNA damage in micronuclei. The findings suggest a new model for the coordination between chromosome segregation and nuclear envelope assembly during normal mitotic exit. The data indicate that these processes are only loosely coordinated through the timing of mitotic spindle disassembly. The absence of precise checkpoint regulation explains why mitotic exit is error-prone, which can explain why chromothripsis is common. Watch this short video to learn more.