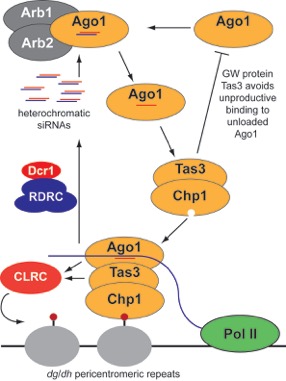
 Small RNA molecules are familiar as negative regulators of endogenous protein-coding genes, but their more deeply conserved function is to ensure genomic stability by keeping repetitive and parasitic elements in check. In the fission yeast Schizosaccharomyces pombe, small RNAs accomplish this task by guiding heterochromatin formation at DNA regions flanking the centromere of each chromosome. The small RNA effector complex that targets the heterochromatin machinery to pericentromeric domains, called RITS, includes an Argonaute protein and a subunit bearing glycine-tryptophan (GW) repeats. The GW motif is a feature of Argonaute-interacting proteins that is conserved across several kingdoms of life and in silencing pathways both nuclear and cytoplasmic. A recent publication from the Moazed Lab has now uncovered a mechanism of ordered assembly for the GW protein-containing RITS complex that prevents Argonautes not yet programmed with a guide RNA from associating with GW repeat-containing proteins. In addition, they show that the poorly characterized fungal protein Arb1 is essential for loading small RNAs onto the S. pombe Argonaute in vitro and for RITS assembly in vivo. Altogether, the results demonstrate that a GW protein can act as a sensor of an Argonaute’s small RNA loading state. Finally, the work suggests that GW proteins play an evolutionarily conserved role in restricting the recruitment of downstream silencing machineries as varied as RNA deadenylases and chromatin-modifying enzymes exclusively to mature, competent Argonaute complexes.
Small RNA molecules are familiar as negative regulators of endogenous protein-coding genes, but their more deeply conserved function is to ensure genomic stability by keeping repetitive and parasitic elements in check. In the fission yeast Schizosaccharomyces pombe, small RNAs accomplish this task by guiding heterochromatin formation at DNA regions flanking the centromere of each chromosome. The small RNA effector complex that targets the heterochromatin machinery to pericentromeric domains, called RITS, includes an Argonaute protein and a subunit bearing glycine-tryptophan (GW) repeats. The GW motif is a feature of Argonaute-interacting proteins that is conserved across several kingdoms of life and in silencing pathways both nuclear and cytoplasmic. A recent publication from the Moazed Lab has now uncovered a mechanism of ordered assembly for the GW protein-containing RITS complex that prevents Argonautes not yet programmed with a guide RNA from associating with GW repeat-containing proteins. In addition, they show that the poorly characterized fungal protein Arb1 is essential for loading small RNAs onto the S. pombe Argonaute in vitro and for RITS assembly in vivo. Altogether, the results demonstrate that a GW protein can act as a sensor of an Argonaute’s small RNA loading state. Finally, the work suggests that GW proteins play an evolutionarily conserved role in restricting the recruitment of downstream silencing machineries as varied as RNA deadenylases and chromatin-modifying enzymes exclusively to mature, competent Argonaute complexes.
(Holoch and Moazed, Nat. Struct. Mol. Biol., PMID 25730778)