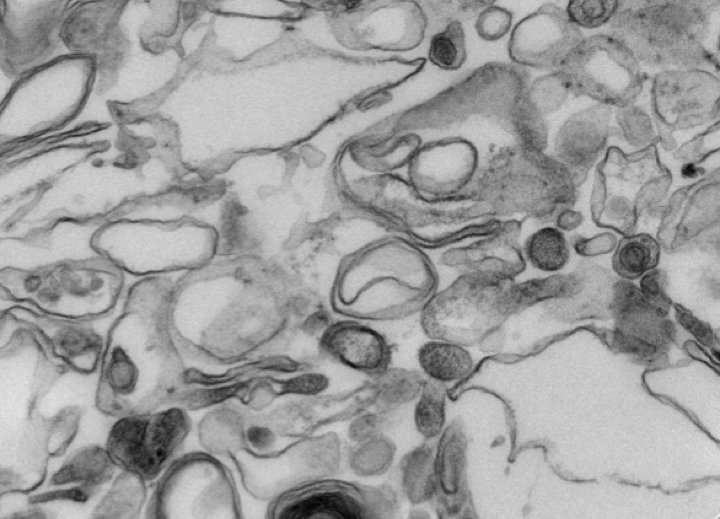
Autophagy, the process by which proteins and organelles are sequestered in double-membrane structures called autophagosomes and delivered to lysosomes for degradation, is critical in diseases such as cancer and neurodegeneration, but our understanding of how cargo is selected and targeted to autophagosomes is incomplete. The Harper Lab in collaboration with Alec Kimmelman’s lab (DFCI) recently reported in Nature the use of quantitative proteomics to systematically identify autophagosome-enriched proteins, including cargo receptors. Among the novel autophagosomally enriched proteins was NCOA4, a cytoplasmic protein that they demonstrated to localize to autophagosomal vesicles in response to activation of autophagy. Unbiased identification of NCOA4-associated proteins revealed ferritin heavy and light chains, components of an iron-filled cage structure that protects cells from reactive iron species but is degraded via autophagy to release iron through an unknown mechanism. They found that delivery of ferritin to lysosomes required NCOA4, and an inability of NCOA4-deficient cells to degrade ferritin leads to decreased bioavailable intracellular iron. Their work identifies NCOA4 as a selective cargo receptor for autophagic turnover of ferritin (ferritinophagy) critical for iron homeostasis and provides a resource for further dissection of autophagosomal cargo-receptor connectivity.
Figure: Electron microscopy of purified autophagosomes employed for quantitative proteomics.