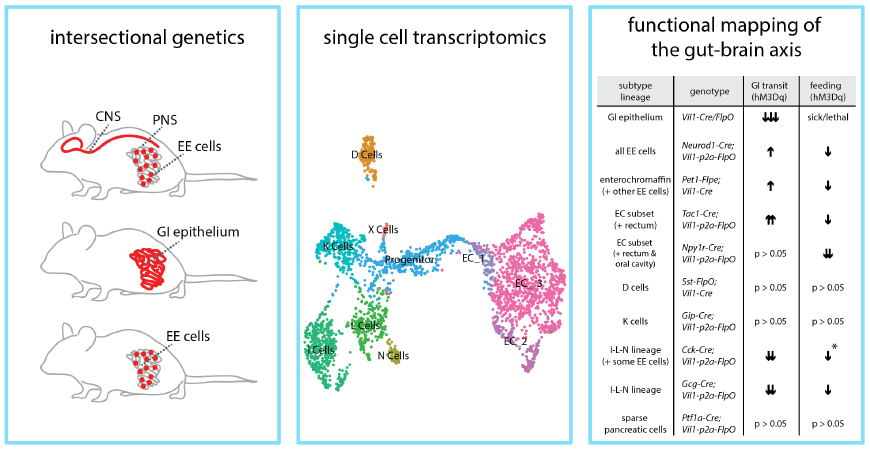
Signals derived from the gut powerfully control many aspects of our physiology and behavior, such as feeding, gastrointestinal physiology, metabolism, and food preference learning. Enteroendocrine cells are specialized sensory cells of the gut-brain axis that are sparsely distributed along the intestinal epithelium. They express a variety of receptors to monitor the intestinal contents and in turn secrete a panel of gut hormones that coordinate physiology and behavior. However, how different enteroendocrine cells successfully orchestrate physiological and behavioral responses is largely unknown. Furthermore, a lack of selective genetic tools has limited molecular and functional annotation of enteroendocrine cells in vivo and hampered our ability to investigate distinct sensory pathways and functional organization of the gut-brain axis.
In this work, Hayashi et al. developed intersectional genetic approaches to obtain highly selective access to enteroendocrine cells in vivo in mice. Authors leveraged chemogenetic approaches and found that in vivo activation of enteroendocrine cells acutely inhibited feeding behavior and accelerated gut motility. Single cell RNA-sequencing experiments revealed diverse enteroendocrine subtypes and their receptor gene expression profiles. Authors then generated a suite of mouse lines that enable highly selective labeling and control of enteroendocrine cell subtypes in vivo. These different transcriptome-defined enteroendocrine cell types variably impacted feeding behavior and gut motility upon acute activation, revealing functional organization of the gut-brain axis. Defining a holistic model for enteroendocrine cell function and their sensory repertoires provides a critical framework for understanding the neuronal and cellular logic underlying how the gut communicates with the brain. Other authors of this work included Judith Kaye, Ella Douglas, and Narendra Joshi of the Liberles lab, and Fiona Gribble and Frank Reimann of University of Cambridge, UK.