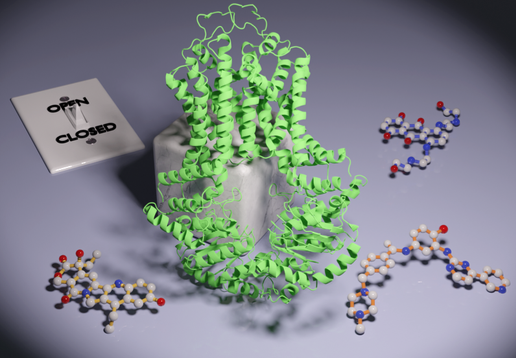
Resistance to chemotherapy is a complication frequently encountered during treatment of difficult recurring cancers. The ability of cancerous cells to resist the cytotoxic effects of chemotherapeutics is often mediated by ABC transporters such as P-glycoprotein, ABCG2, and MRP1, which function to actively pump anti-cancer drugs out of cells. Among these transporters the structure of ABCG2 is unique, with inverted topology and a lack of domain swapping between transmembrane helices. The mechanisms by which ABCG2 recognizes and transports diverse chemotherapy compounds has remained elusive.
In a recent paper published in Nature Communication, the Liao Lab determined a series of cryo-EM structures of ABCG2 bound to different chemotherapy compounds. These structures along with accompanying biochemical assays reveal how anti-cancer drugs induce a conformational switch of the transporter, and how different compounds elicit distinct effects on transporter conformation and function. These results have important implications for future drug development.