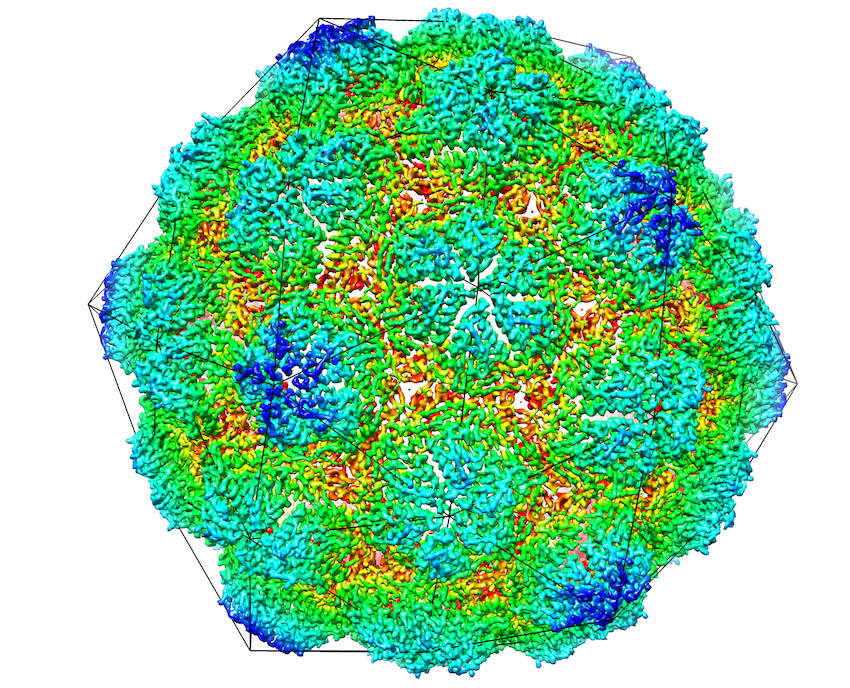
Iron storage proteins are essential for cellular iron homeostasis and redox balance. Ferritin proteins are the major storage units for bioavailable forms of iron. Some organisms lack ferritins, and it is not known how they store iron. In a paper recently published in eLife, the Liao Lab (in collaboration with Dr. Pamela Silver’s lab in the Department of Systems Biology of Harvard Medical School, and Dr. Tobias Giessen at University of Michigan) determines a cryo-EM structure of a 42 nm two-component encapsulin-based iron storage compartment from Quasibacillus thermotolerans. This study reveals the assembly principles of a thermostable T=4 shell topology and its catalytic ferroxidase cargo and show interactions underlying cargo-shell co-assembly. This compartment has an exceptionally large iron storage capacity storing over 23,000 iron atoms, thus revealing a new approach for survival in diverse habitats with limited or fluctuating iron availability via an iron storage system able to store 10 to 20 times more iron than ferritin.