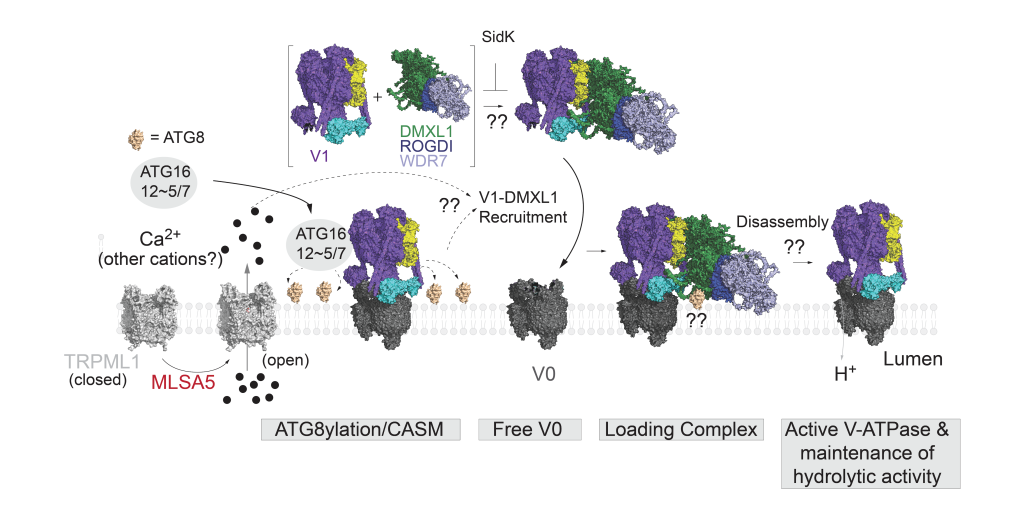
Recent studies have shown that perturbation of the lysosomal proton gradient induces a response that involves conjugation of the autophagy proteins ATG8s (LC3s and GABARAPs) directly onto the lysosomal limiting membrane, a process referred to as CASM. One of the stimuli that induces CASM is activation of a lysosomal ion channel, TPRML1. Interestingly, TRPML1 activation, via small molecule agonists, has been shown to mediate clearance of protein aggregates (e.g. alpha-synuclein), which may confer therapeutic benefits in disease contexts such as Parkinson’s disease. However, how TRPML1 activation and CASM affects the lysosomal proteome had not been explored. In this study, we implemented a lysosome enrichment method, Lyso-IP, coupled with quantitative proteomics, to capture changes in the lysosomal proteome upon TRPML1 activation. Using this approach, we discovered that DMXL1 is recruited to lysosomes in a CASM-dependent manner. Through loss-of-function studies, we demonstrate that DMXL1 supports the recruitment of V1-ATPase, which promotes lysosome acidification and function. Furthermore, using AlphaFold 3 modeling, we identify a LIR (LC3-interacting region) motif in DMXL1, which may mediate direct interaction with ATG8 proteins, and show that it is necessary for lysosomal translocation. Overall, our findings support that TRPML1 activation induces a lysosomal homeostatic response involving CASM-dependent DMXL1-V1-ATPase recruitment.