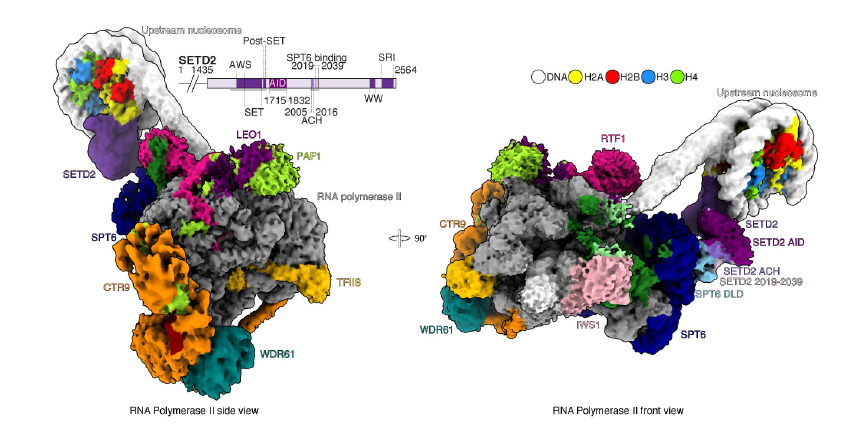
The enzyme SETD2, which deposits H3K36me3 within gene bodies, is frequently mutated in various cancers. While its general function is understood, the mechanistic basis for its gene body-specific activity remains unclear. In this study, we investigate SETD2's mechanism using structural and biochemical approaches, demonstrating that its activity depends on interaction with the transcription elongation machinery, which includes RNA polymerase II and several elongation factors that bind directly to it. We first show how nucleosomes are retained during transcription through a direct interaction between the histone chaperone FACT and the transcription elongation factor RTF1. This interaction tethers FACT, ensuring that histones are retained and re-deposited onto the upstream transcribed DNA. While FACT re-deposits the nucleosome, the elongation factor SPT6 recruits SETD2 and prepares it for H3K36me3 deposition. We further demonstrate that the interaction between SETD2 and SPT6 alleviates SETD2’s auto-inhibition, enabling it to deposit H3K36me3 onto the upstream transcribed nucleosome. Our findings provide the first clear explanation of how transcription elongation factors regulate the epigenetic landscape, offering new insights into SETD2’s mechanism of action.