In a paper published in Nature Structural & Molecular Biology, Henning Arlt (Farese-Walther Lab) and colleagues report the first near full-length structure of seipin.
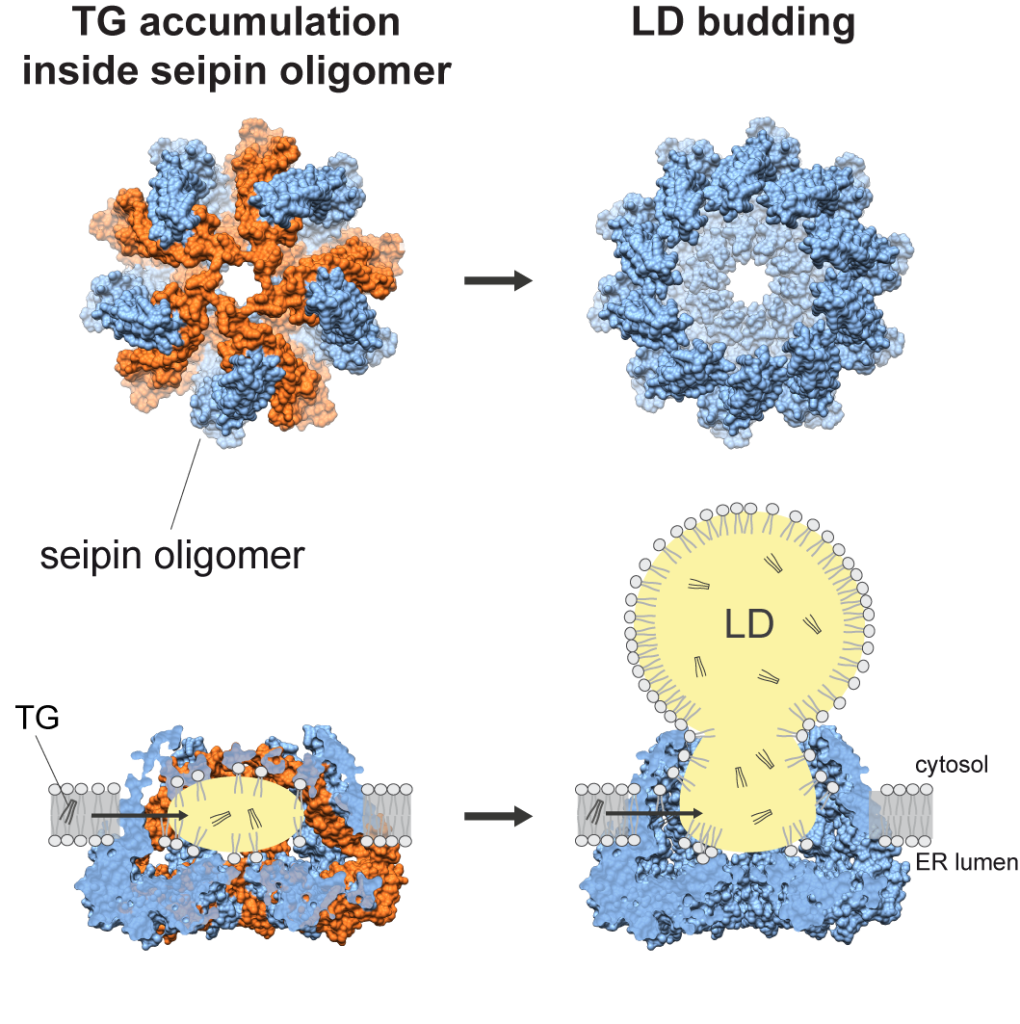
The seipin protein complex is the key machinery for the formation of lipid droplets – cellular organelles that are responsible for storing fats. Previously, the membrane embedded part of the structure had remained elusive, limiting our understanding of how seipin catalyzes lipid droplet formation.\
By applying cryogenic-electron microscopy and structural modeling data, the team of HMS researchers and their collaborators discovered that seipin exists in a cage-like structure and alternates between two conformations. These findings provide clues into the process of lipid droplet formation, suggesting that a closed seipin cage allows for triacylglycerol phase separation and a switch to an open conformation allows for lipid droplet growth and budding.
Importantly, the structure provides a conceptual framework for understanding this elegant protein machinery that governs the process of making oil droplets in cells.