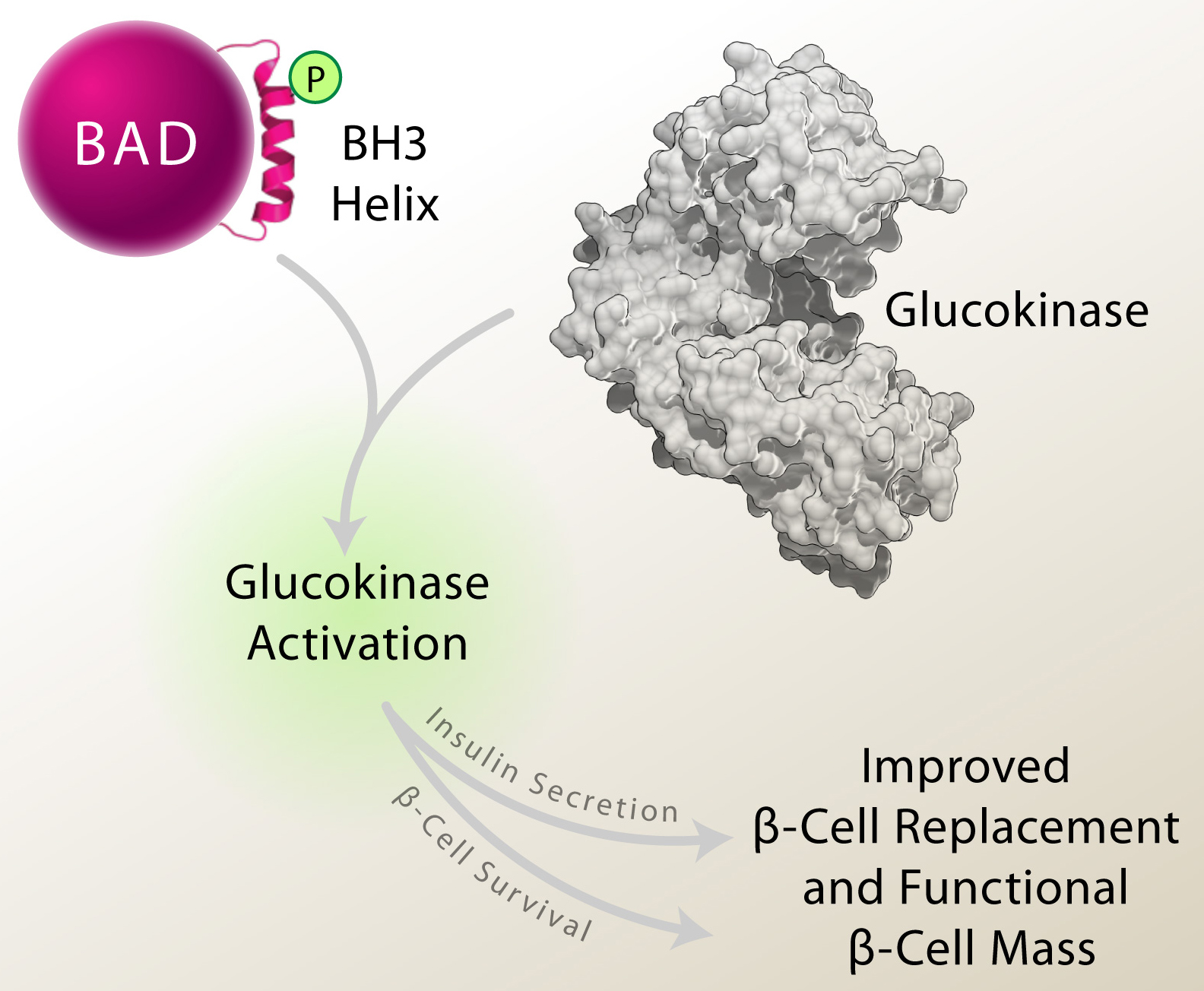
Autoimmune destruction of insulin-producing β-cells leaves individuals with type 1 diabetes (T1D) insulin dependent for life. Strategies that replace or regenerate β-cells, including islet transplantation and β-cell generation from non-β-cell precursors are potential therapies in T1D. However, insufficient β-cell proliferation, survival, and insulin secretory response to glucose can limit the benefits of these strategies. As such, molecular pathways or strategies that simultaneously enhance β-cell mass and glucose signaling are of therapeutic interest. The Danial Lab has identified such strategy. Previous work showed that the BCL-2 family protein BAD stimulates the β-cell glucose response and insulin secretion through phosphorylation of a defined residue within an amphipathic α-helix known as the BCL-2 Homology (BH)-3 domain. This modification neutralizes BAD’s apoptotic function by preventing its capacity to bind and inactivate pro-survival BCL-2, BCL-XL and BCL-w proteins, and simultaneously triggers its ability to directly activate the glucose-metabolizing enzyme glucokinase (GK). However, whether beyond neutralizing BAD’s apoptotic activity, BAD phosphorylation has active, cell autonomous effects on b-cell survival was not known. In the February 2015 issue of Cell Reports, new findings from the Danial Lab show that genetic and pharmacologic approaches to mimic BAD phosphorylation within its BH3 helix not only stimulate insulin secretion but protect β-cells from death induced by T1D-related stress stimuli, including inflammatory, ER and oxidative stress. β-cell survival in this setting is not merely due to the inability of phospho-BAD to suppress pro-survival BCL-2 proteins but requires its activation of GK. Strikingly, the β-cell-autonomous benefits of phospho-BAD mimicry lead to improved donor islet engraftment in transplanted diabetic mice, increased β-cell viability in islet grafts, restoration of insulin release, and diabetes reversal. These findings highlight the utility of BAD phospho-BH3 mimetic approaches in augmenting functional β-cell mass in diabetes.