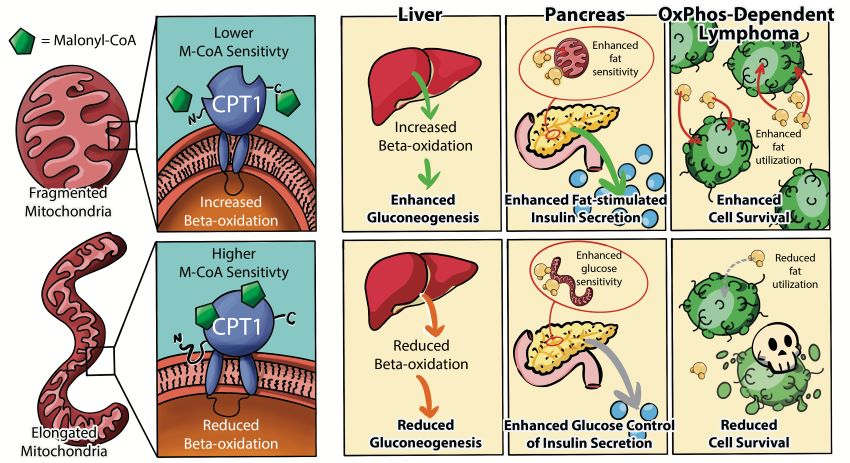
In a collaborative study, Danial lab reveals mitochondrial morphology controls fatty acid oxidation by modulating the sensitivity of CPT1 to its allosteric inhibitor malonyl-CoA.
Cells’ fuel choice can have profound effects on cellular energy, reductive power, and biosynthetic intermediates, thereby influencing cell behavior in myriad of ways. How are cellular fuel decisions established? As key organelles that process and integrate metabolic cues, mitochondria can modulate fuel choices. Increasing evidence also indicates that changes in fuel utilization are associated with changes in mitochondrial membrane dynamics through fusion and fission events. However, whether the relationship between mitochondrial morphology and fuel metabolism is causal and broadly applicable to all fuel substrates or specific for certain fuel decisions remain unknown.
In this study co-led by Danial (DFCI/HMS) and Shirihai (UCLA) labs, a strong linear correlation was found between mitochondrial fragmentation and increased mitochondrial fatty acid oxidation (FAO, β-oxidation) rates across cellular models representing a wide variety of mitochondrial shapes. Forced mitochondrial elongation by genetic manipulation of mitochondrial fusion or fission GTPases selectively diminishes FAO, while forced fragmentation augments FAO without affecting mitochondrial handling of other fuel substrates. Importantly, the link between mitochondrial fragmentation and FAO is not only conserved in multiple cell types from diverse tissue origins but is also required for specific FAO-driven biologic functions relevant to each cell type. These include gluconeogenesis in hepatocytes, adaptive increases in β-cell insulin secretion in response to obesity, and survival of FAO-dependent cancer cells. Remarkably, the effect of fragmentation on FAO is selective for long-chain FAO, pointing to carnitine palmitoyltransferase 1 (CPT1) as the downstream effector of mitochondrial morphology in regulation of FAO. Mechanistically, fragmentation reduces malonyl-CoA inhibition of CPT1, while elongation increases CPT1 sensitivity to malonyl-CoA inhibition. This work underscores a physiologic role for mitochondrial fragmentation as a basic mechanism whereby cellular fuel preference and FAO capacity are determined.