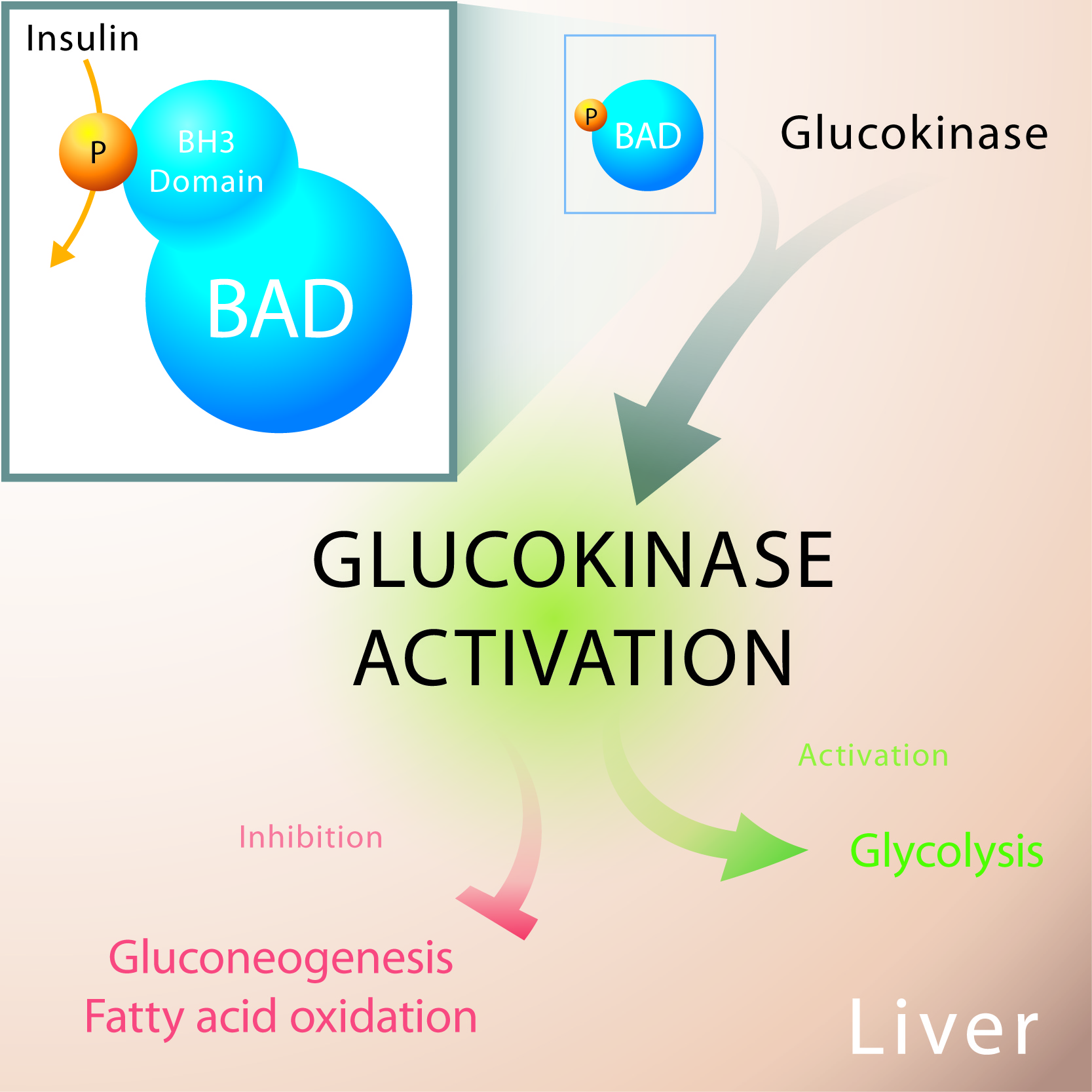
The homeostatic balance of hepatic glucose utilization, storage and production is exquisitely controlled by hormonal signals and hepatic carbon metabolism during fed and fasted states. How the liver senses extracellular glucose to cue glucose utilization versus production is not fully understood. During short term fasting, glucose is produced by both net glycogenolysis and gluconeogenesis, whereas upon prolonged fasting, glucose is synthesized almost exclusively from gluconeogenesis. Abnormal elevation of hepatic glucose production is a chief determinant of fasting hyperglycemia in diabetes. In the February 2014 issue of Cell Metabolism, the Danial Lab reported that the physiologic balance of hepatic glycolysis and gluconeogenesis is regulated by BAD, a dual function protein with roles in apoptosis and metabolism. BAD deficiency reprograms hepatic substrate and energy metabolism towards diminished glycolysis, excess fatty acid oxidation and exaggerated glucose production that escapes suppression by insulin. The group conducted genetic and biochemical studies that revealed BAD’s suppression of gluconeogenesis is actuated by phosphorylation of its BH3 domain and subsequent activation of the glucose-phosphorylating enzyme glucokinase. They also found BAD-GK axis is required for suppression of hepatic glucose production by insulin. The physiologic relevance of these findings is evident from the ability of a BAD phospho-mimic variant to counteract unrestrained gluconeogenesis and improve glycemia in leptin resistant and high-fat diet models of diabetes and insulin resistance. These findings mark BAD as a regulator of hepatic substrate metabolism and insulin sensitivity.