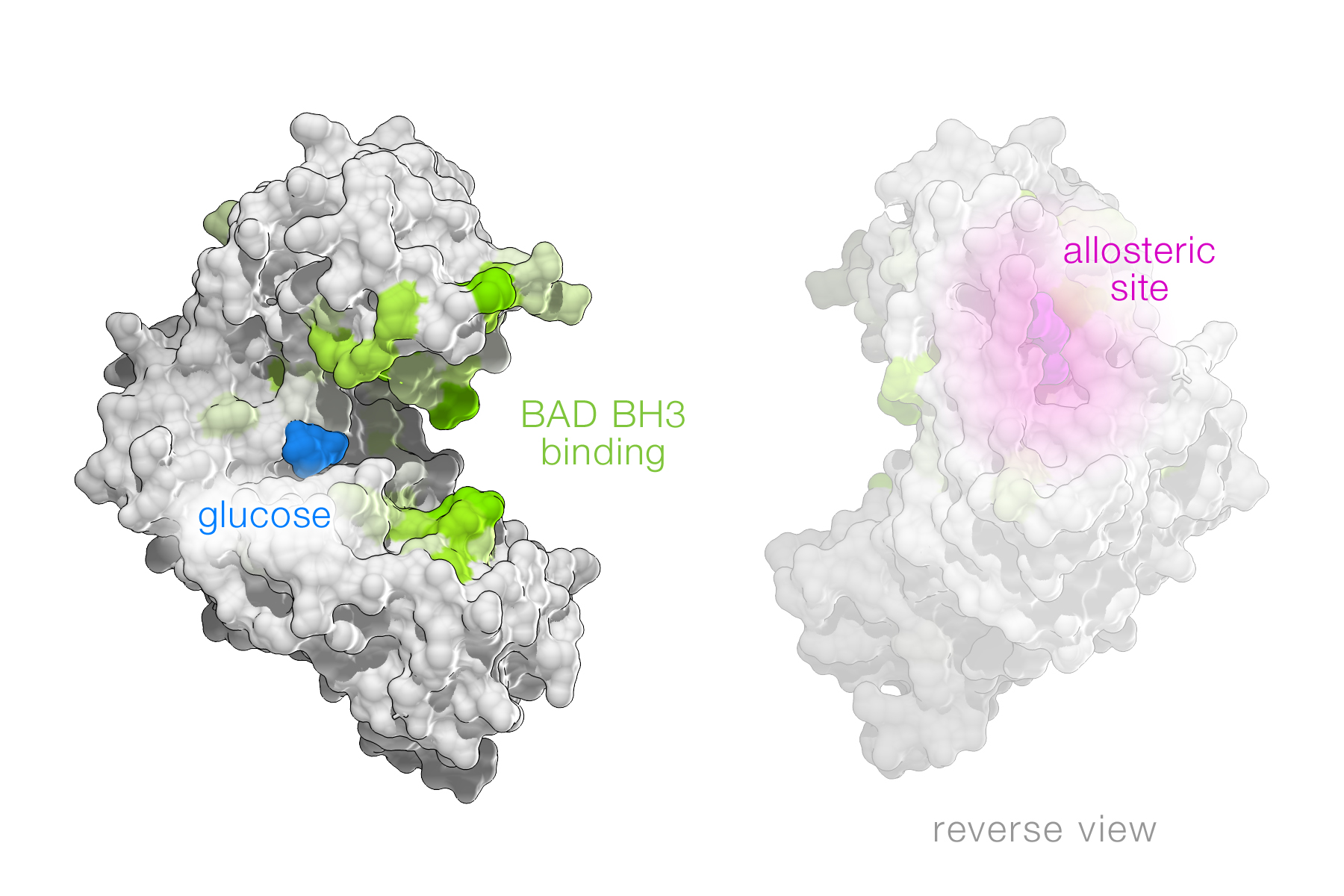
Glucokinase is a glucose-phosphorylating enzyme that regulates insulin release and hepatic metabolism, and its loss-of-function is implicated in the pathogenesis of diabetes. Glucokinase activators (GKAs) are attractive therapeutics in diabetes, however, clinical data indicate that their benefits can be offset by hypoglycemia due to marked allosteric enhancement of the enzyme’s glucose affinity. Recent collaborative studies between the Danial and Walensky laboratories led to the discovery of a novel class of GKAs. These findings were published in the January 2014 issue of Nature Structural & Molecular Biology and showed that the BCL-2 homology 3 (BH3) alpha-helix derived from human BAD, a glucokinase binding partner, increases the enzyme catalytic rate without dramatically changing glucose affinity. This provides a new mechanism for pharmacologic activation of glucokinase. Remarkably, BAD BH3 phospho-mimetic mediates these effects by engaging a novel region near the enzyme’s active site. This interaction increases insulin secretion in human islets and restores the function of naturally-occurring human glucokinase mutants at the active site. Thus, BAD phospho-mimetics may serve as a novel class of GKAs.
The figure demonstrates the interaction region of the BAD BH3 helix near the glucokinase active site where glucose binds. This site is distinct from the allosteric site of the enzyme shown in the reverse view.