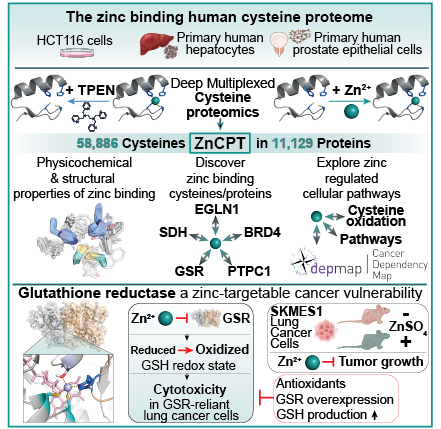
Zinc is an essential micronutrient that regulates a wide range of physiological processes, principally through zinc binding to protein cysteine residues. Despite being critical for modulation of protein function, for the vast majority of the human proteome, the cysteine sites subject to zinc binding remain undefined. To address this, we developed ZnCPT, a deep and quantitative mapping of the zinc binding cysteine proteome that quantifies the zinc binding status across over 58,000 cysteine sites. We define 6173 zinc binding protein cysteines, uncovering protein families across major domains of biology that are subject to either constitutive or inducible zinc binding. The systematic structural analysis of zinc binding site environments identified distinct features providing a structural basis for constitutive and inducible zinc coordination, including physicochemical determinants. Cross-referencing ZnCPT with published redox proteome data revealed that most cysteines belong to distinct subpopulations, either redox regulated or zinc binding, enhancing our understanding of cysteine functionality. ZnCPT further enables systematic discovery of zinc-regulated structural, enzymatic, and allosteric functional domains. On this basis, we identify 52 cancer genetic dependencies subject to zinc binding and nominate malignancies sensitive to zinc-induced cytotoxicity. In doing so, we discover a mechanism of zinc regulation over Glutathione Reductase (GSR) that drives cell death in GSR-dependent lung cells and tumors in vivo. Mechanistic analyses revealed zinc-mediated inhibition of GSR by binding to its active site which leads to disruption of cellular redox homeostasis and pronounced cytotoxicity that can be alleviated through pharmacological or genetic elevation of the antioxidant thiol pool. Furthermore, zinc supplementation significantly reduced the growth of GSR-dependent lung cancer cells in a murine tumor xenograft model. We provide ZnCPT as a resource for understanding mechanisms of zinc regulation of protein function.