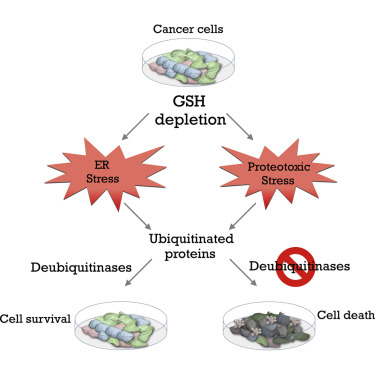
 A longstanding dogma is that antioxidants have the ability to aid in preventing cancer development. Recently, this dogma has been called into question because many experimental studies and clinical trials have demonstrated that antioxidants can promote, rather than retard, the development of cancer. In addition, antioxidants can reduce the efficacy of cancer therapies. Therefore, it is critical to understand which cellular antioxidants are involved in these effects and how to block them to prevent cancer progression and improve cancer therapies. In a recent paperpublished in Cell Metabolism, Dr. Isaac Harris, a postdoctoral fellow in the Brugge lab, and his collaborators examined the role of the most abundant endogenous cellular antioxidant, glutathione, in survival of cancer cells. Although this metabolite was identified more than 130 years ago, its role in cancer cells and their response when its production is impaired are still poorly understood. A large number of cell lines from different cancer types (e.g. breast, lung, ovarian) were tested with an inhibitor of glutathione synthesis. Cancer cells were found to be remarkably resistant to any deleterious effects associated with depletion of this antioxidant. To explore how cancer cells could survive without this abundant antioxidant, Dr. Harris and colleagues utilized high-throughput genetic and pharmacologic screens to identify cellular factors that are required for resistance to glutathione depletion. These screens identified a family of proteins, termed deubiquitinases (DUBs), which play a crucial role in maintaining survival of cancer cells after inhibition of glutathione synthesis. Importantly, combined inhibition of DUBs and glutathione production led to a profound induction of death in cancer cells. These findings shed light into the roles of antioxidants in cancer cells and provide a groundwork for novel cancer therapeutics in the future.
A longstanding dogma is that antioxidants have the ability to aid in preventing cancer development. Recently, this dogma has been called into question because many experimental studies and clinical trials have demonstrated that antioxidants can promote, rather than retard, the development of cancer. In addition, antioxidants can reduce the efficacy of cancer therapies. Therefore, it is critical to understand which cellular antioxidants are involved in these effects and how to block them to prevent cancer progression and improve cancer therapies. In a recent paperpublished in Cell Metabolism, Dr. Isaac Harris, a postdoctoral fellow in the Brugge lab, and his collaborators examined the role of the most abundant endogenous cellular antioxidant, glutathione, in survival of cancer cells. Although this metabolite was identified more than 130 years ago, its role in cancer cells and their response when its production is impaired are still poorly understood. A large number of cell lines from different cancer types (e.g. breast, lung, ovarian) were tested with an inhibitor of glutathione synthesis. Cancer cells were found to be remarkably resistant to any deleterious effects associated with depletion of this antioxidant. To explore how cancer cells could survive without this abundant antioxidant, Dr. Harris and colleagues utilized high-throughput genetic and pharmacologic screens to identify cellular factors that are required for resistance to glutathione depletion. These screens identified a family of proteins, termed deubiquitinases (DUBs), which play a crucial role in maintaining survival of cancer cells after inhibition of glutathione synthesis. Importantly, combined inhibition of DUBs and glutathione production led to a profound induction of death in cancer cells. These findings shed light into the roles of antioxidants in cancer cells and provide a groundwork for novel cancer therapeutics in the future.
This work was funded by the Ludwig Cancer Research, Susan G. Komen for the Cure and Canadian Institutes of Health Research (CIHR).